Abstract
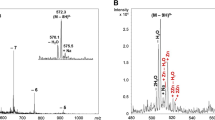
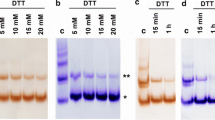
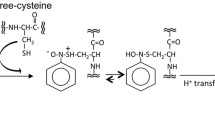
The Zn(II) binding by partial peptides of human protamine HP2: HP21–15; HP21–25, HP226–40, HP237–47, and HP243–57 was studied by circular dichroism (CD). Precipitation of a 20‐mer DNA by these partial peptides and the effects of Zn(II) thereon were investigated using polyacrylamide gel electrophoresis (GE). The results of this study suggest that reduced HP2 (thiol groups intact) can bind Zn(II) at various parts of the molecule. In the absence of DNA, the primary Zn(II) binding site in reduced HP2 is located in the 37–47 sequence (involving Cys‐37, His‐39, His‐43, and Cys‐47), while in the presence of DNA, the strongest Zn(II) binding is provided by sequences 12–22 (by His‐12, Cys‐13, His‐19, and His‐22) and 43–57 (His‐43, Cys‐47, Cys‐53, and His‐57). In its oxidized form, HP2 can bind zinc through His residues of the 7–22 sequence. Zn(II) markedly enhances DNA binding by all partial peptides. These findings suggest that Zn(II) ions may be a regulatory factor for sperm chromatin condensation processes.
Similar content being viewed by others
Reference
McKay DJ, Renaux BS, Dixon GH: Human sperm protamines. Aminoacid sequences of two forms of protamine P2. Eur J Biochem 156: 5–8, 1986
Ammer H, Henschen A, Lee CH: Isolation and amino-acid sequence analysis of human sperm protamines P1 and P2. Occurrence of two forms of protamine P2. Biol Chem Hoppe-Seyler 367: 515–522, 1986
Pogany GC, Corzett M, Weston S, Balhorn R: DNA and protein content of mouse sperm. Exp Cell Res 136: 127–136, 1981
Balhorn R, Reed S, Tanphaichitr N: Aberrant protamine 1/protamine 2 ratios in sperm of infertile human males. Experientia 44: 52–55, 1988
Bjorndahl L, Kvist U: Influence of seminal vesicular fluid on the zinc content of human sperm chromatin. Int J Androl 13: 232–237, 1990
Bench G, Corzett MH, Kramer CE, Grant PG, Balhorn R: Zinc is sufficiently abundant within mammalian sperm nuclei to bind stoichiometrically with protamine 2. Mol Reprod Dev 56: 512–519, 2000
Gatewood JM, Schroth GP, Schmid CW, Bradbury EM: Zinc-induced secondary structure transitions in human sperm protamines. J Biol Chem 265: 20667–20672, 1990
Bianchi F, Rousseaux-Prevost R, Sautiere P, Rousseaux J: P2 protamines from human sperm are zinc-finger proteins with one Cys2/His2 motif. Biochem Biophys Res Commun 182: 540–547, 1992
Quintanilla-Vega B, Hoover DJ, Bal W, Silbergeld E, Waalkes MP, Anderson LD: Lead interaction with human protamine HP2 as a mechanism of male reproductive toxicity. Chem Res Toxicol 13: 594–600, 2000
Kozlowski H, Bal W, Dyba M, Kowalik-Jankowska T: Specific structure-stability relations in metallopeptides. Coord Chem Rev 184: 319–346, 1999
Bal W, Jeżowska-Bojczuk M, Kasprzak KS: Binding of nickel(II) and copper(II) to the N-terminal sequence of human protamine HP2. Chem Res Toxicol 10: 906–914, 1997
Bal W, Lukszo J, Kasprzak KS: Mediation of oxidative DNA damage by nickel(II) and copper(II) complexes with the N-terminal sequence of human protamine HP2. Chem Res Toxicol 10: 915–921, 1997
Liang R, Senturker S, Shi X, Bal W, Dizdaroglu M, Kasprzak KS: Effects of Ni(II) and Cu(II) on DNA interaction with the N-terminal sequence of human protamine P2: Enhancement of binding and mediation of oxidative DNA strand scission and base damage. Carcinogenesis 20: 893–898, 1999
Bal W, Wójcik J, Maciejczyk M, Grochowski P, Kasprzak KS: Induction of a secondary structure in the N-terminal pentadecapeptide of human protamine HP2 through Ni(II) coordination. An NMR study. Chem Res Toxicol 13: 823–830, 2000
Bianchi F, Rousseaux-Prevost R, Bailly C, Rousseaux J: Interaction of human P1 and P2 protamines with DNA. Biochem Biophys Res Commun 201: 1197–1204, 1994
Krizek BA, Merkle DL, Berg JM: Ligand variation and metal ion binding specificity in zinc finger peptides. Inorg Chem 32: 937–940, 1993
Berg JM, Godwin HA: Lessons from zinc-binding peptides. Annu Rev Biophys Biomol Struct 26: 357–371, 1997
Meienhofer J, Waki M, Heimer EP, Lambros TJ, Makofske RC, Chang CD: Solid phase synthesis without repetitive hydrolysis. Preparation of leucylalanyl-glycyl-valine using 9-fluorenylmethyloxy-carbonylamino acids. Int J Peptide Prot Res 13: 35–42, 1979
Irving H, Miles MG, Pettit LD: A study of some problems in determining the stoichiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal Chim Acta 38: 475–488, 1967
Gans P, Sabatini A, Vacca A: SUPERQUAD: An improved general program for computation of formation constants from potentiometric data. J Chem Soc Dalton Trans 1195–1199, 1985
Lees WJ, Whitesides GM: Equilibrium constants for thiol-disulfide interchange reactions: A coherent, corrected set. J Org Chem 58: 642–647, 1993
Krężel A, Leśniak W, Jeżowska-Bojczuk M, Młynarz P, Brasuń J, Kozłowski H, Bal W: Coordination of heavy metals by dithiothreitol, a commonly used thiol group protectant. J Inorg Biochem 84: 77–88, 2001
Banerjea D, Kaden T, Sigel H: Enhanced stability of ternary complexes in solution through the participation of heteroaromatic N bases. Comparison of the coordination tendency of pyridine, imidazole, ammonia, acetate and hydrogen phosphate toward metal ion nitrilotriacetate complexes. Inorg Chem 20: 2586–2590, 1981
Vu HM, Minch MJ: α-(Ac)AKRHRKV, a model of the histone H4 amino terminus, uses an unprotonated histidine in phosphate binding. J Pep Res 51: 162–170, 1998
Woody RW: Circular dichroism and conformation of unordered peptides. Adv Biophys Chem 2: 37–79, 1992
Roehm PC, Berg JM: Sequential metal binding by the RING finger domain of BRCA1. Biochemistry 36: 10240–10245, 1997
Basile LA, Coleman JE: Optical activity associated with the sulfur to metal charge transfer bands of Zn and Cd GAL4. Prot Sci 1: 617–624, 1992.
Vašák M, Kägi JHR, Hill HAO: Zinc(II), cadmium(II) and mercury(II) thiolate transitions in metallothionein. Biochemistry 20: 2852–2856, 1981
Worthington MT, Amann BT, Nathans D, Berg JM: Metal binding properties and secondary structure of the zinc-binding domain of Nup475. Proc Natl Acad Sci USA 93: 13754–13759, 1996
Posewitz MC, Wilcox DE: Properties of the Sp1 zinc finger 3 peptide: Coordination chemistry, redox reactions, and metal binding competition with metallothionein. Chem Res Toxicol 8: 1020–1028, 1995
Predki PF, Sarkar B: Effect of replacement of ‘zinc finger’ zinc on estrogen receptor DNA interactions. J Biol Chem 267: 5842–5846, 1992
Fita I, Campos JL, Puigjaner LC, Subirana, JA: X-ray diffraction study of DNA complexes with arginine peptides and their relation to nucleoprotamine structure. J Mol Biol 167: 157–177, 1983
Balhorn R: A model for the structure of chromatin in mammalian sperm. J Cell Biol 93: 298–305, 1982
Hud NV, Milanovich FP, Balhorn R: Evidence of novel secondary structure in DNA-bound protamine is revealed by Raman spectroscopy. Biochemistry 33: 7528–7535, 1994
Wallace TJ, Schriesheim A: Solvent effects in the base-catalyzed oxidation of mercaptans with molecular oxygen. J Org Chem 27: 1514–1516, 1962
Kvist U: Importance of spermatozoal zinc as temporary inhibitor of sperm nuclear chromatin decondensation ability in man. Acta Physiol Scand 109: 79–84, 1980
Bjorndahl L, Kjellberg S, Roomans GM, Kvist U: The human sperm nucleus takes up zinc at ejaculation. Int J Androl 9: 77–80, 1986
Kvist U, Bjorndahl L, Kjellberg S: Sperm nuclear zinc, chromatin stability, and male fertility. Scanning Microsc 1: 241–1247, 1987
Kvist U, Kjellberg S, Bjorndahl L, Soufir JC, Arver S: Seminal fluid from men with agenesis of the Wolffian ducts: Zinc-binding properties and effects on sperm chromatin stability. Int J Androl 13: 245–252, 1990
Balhorn R, Corzett M, Mazrimas JA: Formation of intraprotamine disulfides in vitro. Arch Biochem Biophys 296: 384–393, 1992
Zalensky AO, Allen MJ, Kobayashi A, Zalenskaya IA, Balhorn R, Bradbury EM: Well-defined genome architecture in the human sperm nucleus. Chromosoma 103: 577–590, 1995
Allen MJ, Bradbury EM, Balhorn R: AFM analysis of DNA-protamine complexes bound to mica. Nucleic Acids Res 25: 2221–2226, 1997
Brewer, LR, Corzett M, Balhorn R: Protamine-induced condensation and decondensation of the same DNA molecule. Science 286: 120–123, 1999
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bal, W., Dyba, M., Szewczuk, Z. et al. Differential zinc and DNA binding by partial peptides of human protamine HP2. Mol Cell Biochem 222, 97–106 (2001). https://doi.org/10.1023/A:1017971525105
Issue Date:
DOI: https://doi.org/10.1023/A:1017971525105