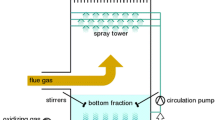
Abstract
Hydrogen sulfide– containing natural gases are considered a fundamental source for obtaining elemental sulfur due to depletion of natural reserves. The basic industrial method of extraction of hydrogen sulfide from natural gases with production of elemental sulfur is the Claus method. The method of partial catalytic oxidation of hydrogen sulfide, which does not require preliminary treatment of the gases and concentration of the hydrogen sulfide, is more promising. This method is being increasingly used in industry.
Similar content being viewed by others
REFERENCES
US Patent No. 5494869 (1996).
Russian Patent No. 2001 677 (1993).
T. G. Alkhazov and N. S. Amirgulyan, Sulfur Compounds in Natural Gases and Crude Oils [in Russian], Nedra, Moscow (1989).
F. R. Ismagilov, S. R. Khairulin, N. M. Dobrynkin, et al., Prospects for Utilization of Hydrogen Sulfide in Oil Refineries by Direct Heterogeneous Oxidation into Sulfur [in Russian], TsNIITEneftekhim, Moscow (1991).
French Patent No. 2103058 (1995).
Russian Patent No. 2070086 (1996).
T. G. Alkhazov and A. A. Vartanov, Izv. Vyssh. Uchebn. Zaved., Neft' Gaz, No. 2, 41-44 (1979).
Russian Patent No. 2070089 (1996).
Russian Patent No. 2094114 (1997).
S. R. Khairulin, Z. R. Ismagilov, and M. A. Kerznehtsev, Khim. Promyshl., No. 4, 53-56 (1996).
F. R. Ismagilov and F. M. Latypova, Neftepererab. Neftekhim., No. 11, 25-27 (1997).
F. R. Ismagilov, A. V. Podshivalin A.V. Balaev, et al., Gaz. Promyshl., No. 4, 21-23 (1993).
T. G. Alkhazov and N. S. Amirgulyan, Kinet. Katal., No. 5, 1130-1134 (1982).
French Patent Application No. 2727101 (1996).
Russian Patent No. 1829 182 (1995).
Russian Inventor's Certificate No. 1582537 (1994).
Russian Patent No. 2035221 (1995).
N. M. Dobrynkin, A. A. Davydov, M. V. Batygina, et al., Zh. Fiz. Khim., 72, No. 6, 1027-1030 (1998).
USSR Inventor's Certificate No. 1398304 (1993).
K. T. Li and N. Shyu, Ind. Eng. Chem. Res., No. 5, 1480-1484 (1997).
V. G. Lipovich, M. A. Kapustin, T. Yu. Belova, et al., Khim. Tverd. Topl., No. 4, 134-137 (1991).
A. F. Lunin, A. Kh. Khussein, T. N. Burdeinaya, et al., Khim. Tekhnol. Topl. Masel, No. 12, 13 (1993).
V. I. Marshneva and V. V. Mokrinskii, Kinet. Katal., 29, No. 4, 989-993 (1988).
S. R. Khairulin, Candidate Dissertation, Institute of Industrial Chemistry, Ukrainian Branch, Russian Academy of Sciences, Perm' (1998).
Russian Patent No. 2110324 (1998).
G. G. Kuvshinov, Yu. M. Mogil'nykh, and M. Yu. Lebedev, Khim. Promyshl., No. 1, 28-35 (1999).
L. Dudzik and K. F. Preston, Int. Chem. Eng., No. 1, 35 (1967).
R. Sreeramamuthy and P. G. Menon, J. Catal., 37, No. 2, 287 (1975).
O. C. Cariaso and P. L. Walker Jr., Carbon, No. 3, 233 (1975).
J. Klein and K.-D. Henning, Fuel, No. 8, 1064 (1984).
R. M. Barrer and S. Wasilevski, Roczn. Chem., 35, No. 4, 999 (1961).
Pan Zhenglu, Hung-Shan Weng, Feng Han-Yu, et al., AlChE J.y, 30, No. 6, 1021-1024 (1984).
T. G. Alkhazov and N. S. Amirgulyan, in: Proceedings of the VII Sino-Soviet Seminar on Catalysis, June 18-21, 1983 [in Russian], Izd. IK SO RAN, Novosibirsk (1983), p. 238.
Yu. N. Brodskii, V. I. Gerus, S. M. Golyand, et al., Gaz. Promyshl., No. 8, 42 (1966).
Y. Iwasawa and S. J. Ogasawara, J. Catal., 46, No. 3, 345 (1977).
N. S. Amirgulyan, Candidate Dissertation, Az. Institute of Petrochemistry, Baku (1982).
A. N. Zagoruiko and V. V. Mokrinskii, in: Proceedings of the XIV International Conference on Chemical Reactors, June 23-26, 1998, Tomsk [in Russian], IK SO RAN, Novosibirsk (1998), pp. 155-156.
Kuo-Tseng Li, Ni-Shen Shyu, Ind. Eng. Chem. Res., 36, 1480-1484 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Konshenko, E.V., Balaev, A.V., Ismagilov, F.R. et al. Direct Catalytic Oxidation of Hydrogen Sulfide. Chemistry and Technology of Fuels and Oils 37, 212–218 (2001). https://doi.org/10.1023/A:1017941209151
Issue Date:
DOI: https://doi.org/10.1023/A:1017941209151