Abstract
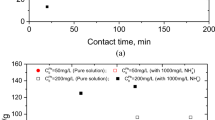
This study records experiments undertaken to determine the suitable conditions for the use of naturally occurring minerals (talc, chalcopyrite and barite) as an adsorbent for the removal of lead ions from liquid wastes. The adsorption of lead ions from solutions containing different initial lead concentrations (50, 100, 200, 400, 600, 800 and 1000 mg l−1 Pb as lead nitrate) using different size fractions (<63 μm, 63–150 μm) of talc, chalcopyrite and barite at different pH (3, 5, 7 and 9) and different adsorption times (24, 48, 72 and 96 hr) was examined. The results revealed that of the studied minerals, the chalcopyrite fraction at 63–150 μm showed the highest adsorption capacity. The adsorption data of Pb ions was also analyzed with the help of the Langmuir and Freundlich models to evaluate the mechanistic parameters associated with the adsorption process. The adsorption isotherms obtained from the Langmuir and Freundlich equations were generally linear and the adsorption of Pb by the studied minerals was correlated with the adsorption maximum and binding energy constant of the Langmuir equation and equilibrium partition constant and binding partition coefficient of the Freundlich equation. It was concluded that the equilibrium time of adsorption was 72 hr at an optimum pH from 7 to 9. This technique might be successfully used for the removal of lead ions from liquid industrial wastes and wastewater.
Similar content being viewed by others
References
Ajmal, M., Rao, R.A.K. and Slddiqui, B.A. (1995) Adsorption studies and removal of dissolved metals using pyrolusite as adsorbent. Environmental Monitoring and Assessment 38, 25–35.
Bargar, J., Brown, G.E., Jr. and Parks, G.A. (1998) Surface complexation of Pb(II) at oxide-water interfaces: III. XAFS Determination of Pb(II)and (PbII)-chloro adsorption complexes on goethite and alumina. Geochimstry and Cosmochimtry Acta 62(2), 195–207.
Bohn H., McNeal, B. and Conner, G.O. (1985) Soil chemistry. New York: John Wiley and Sons.
Campbell, L.S. and Davies, B.E. (1995) Soil sorption of cesium modeled by the Langmuir and Freundlich isotherm equation. Applied Geochemistry, 10, 715–23.
Dabrowski, A. and Tertykh, V.A. (1992) Adsorption on new and modified inorganic sorbents, London: Elsevier Science, Ltd.
Davis, J.A. and Kent, D.B. (1990) Surface complexation modeling in aqueous geochemistry. In (M.F. Hochella, Jr. and A.F. White, eds) Mineral-water interface geochemistry, Rev. Mineral 23, 177–305.
Duker, A., Ledin, A., Karlsson, S. and Allard, B. (1995) Adsorption of zinc on colloidal(hydr) oxides of Si, Al and Fe in the presence of a fulvic acid. Applied Geochemistry 10, 197–205.
Gadde, R.R. and Laitinen, H.A. (1974) Study of the sorption of lead by hydrous manganese oxide. Analytical Chemistry 46, 2022–2026.
Gardea-Torresdey, J.L., Tiemann, K.J., Gonzalez, J.H. and Rodrignez, O. (1996) Biosorption of cadmium, chromium, lead and zinc by biomass of Medicago sativa (Alfalfa). In HSRC/WERC Joint Conference on the Environment, USA.
Girgis, B.S. and Hendawy, A.N.A. (1997) Capacity of activated carbon from date pips in the removal of organic pollutants and heavy metals. In 1st international conference on chemistry education, Cairo, Egypt, pp. 55–62.
Gunnerinsson, L., Lovgren, L. and Sjoberg, S. (1994) Complexation of PB(II) at the goethite (FeOOH)/water interface: the influence of chloride. Geochimistry and Cosmochimistry Acta 58, 4973–83.
Hall, M.J. (1998) Kaolinite sorbent for the removal of heavy metals from incinerated lubricating oils. Project, University of Texas.
Hunng, C.P., Elliot, H.A. and Ashmead, R.M. (1997) Interfacial reaction and the fate of heavy metals in soilwater system, Journal of Water Pollution Control Fed. 49, 745–56.
Jain, C.K. and Ram, D. (1997) Adsorption of lead and zinc on bed sediments of the river Kali. Water Research 31(1), 154–63.
Jenne, E.A. and Zachara, J. (1982) Factors influencing the sorption of metals In Waste in the ocean, vol. 2, Dredgedmaterial disposal in the ocean (D.R. Kester, B.H. Ketchum, I.W. Duedall and P.K. Parks, eds), pp. 83–98. New York: John Wiley and Sons.
Koshima, H. and Onishi, H. (1986) Adsorption of metal ions on activated carbon from aqueous solution at pH 1–13. Talanta 33, 391–95.
Langmuir, I. (1918) The adsorption of gases on plane surface of glass, mica and platinum, American Chemical Society 40, 1361–1403.
Lin, Z. and Puls, R.W. (2000) Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environmental Geology 39, 725–59.
Muscas, L. (1995) Adsorption at transport of lead in porous media. In European conference for young researchers in chemical engineering 1, 182–84.
Nowack, B. and Sigg, L. (1996) Adsorption of EDTA and metal-EDTA complexes to goethite, Journal of Colloid Interfaces Science 177, 106–121.
Reeder, R.J. (1996) Interaction of divalent cobalt, zinc, cadmium and barium with the calcite surface during layer growth. Geochimistry and Cosmochimistry Acta 60, 1543–52.
Sigworth, E.A. and Smith, S.B. (1972) Adsorption of inorganic compound by activated carbon. Journal American Water Wks Ass. 64, 386–91.
Smith, E.A. (1992) Heavy metals, pp. 142–50. WA: Health Science Bookstall.
Srivestava, S.K., Tyagi, R. and Paut, N. (1989) Adsorption of heavy metals on carbonaceous material developed from the waste slurry generated in a local fertilizer plant. Water Research 23(9), 1161–65.
Strong, B. (2000) Coffee removes heavy metals from tap water http://www.beyond 2000.com/news/story–427.html.
Sturchio, N.J., Chiarello, R.P., Cheng, L., Lyman, P.F., Bedzyk, M.J., Qian, Y., You, H. and others. (1997) Lead adsorption at the calcite-water interface, Geochimistry and Cosmochimistry Acta 61(2), 251–63.
Tokunaga, S. and Uthium, A. (1997) Survey on advanced treatment of arsenic-lead contaminating wastewater. Journal NIMC 5, 21–38.
Weber, W.J., Jr. (1972) Physicochemical processes for water quality control, pp. 208–10. New York: John Wiley and Sons.
Yuan, G., Seyama, H., Soma, M., Theng, B.K.G. and Tanaka, A. (1999) Adsorption of some heavy metals by natural zeolites. Environmental Science and Health part A 34(3), 625–48.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rashed, M.N. Lead removal from contaminated water using mineral adsorbents. The Environmentalist 21, 187–195 (2001). https://doi.org/10.1023/A:1017931404249
Issue Date:
DOI: https://doi.org/10.1023/A:1017931404249