Abstract
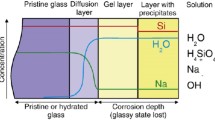
Numerical simulations of the water dissolution of a random ternary solid are presented. The three elements represent silica, soluble oxides (alkalis and boron) and quasi-insoluble oxides (Al2O3, ZrO2, Fe2O3,...). The soluble species are dissolved immediately when they are in contact with the solution. Their proportion is kept below the percolation threshold. For the other species, one introduces a model of dissolution-recondensation. It is shown that the dissolution rate constants should be dependent on the bonding environment in order to include surface tension. The condensation fluxes are proportional to the concentration of each species in solution. In the dynamic regime (no recondensation), one observes the congruent dissolution of silica and soluble species, after a short initial phase of selective extraction of the soluble species. The common rate of dissolution decreases with the proportion of insoluble species and increases sharply with that of soluble species. This is mainly due to the formation of a porous hydrated layer whose active surface area increases markedly with the proportion of soluble species. In the static regime (finite solution volume), the equilibrium solubility of silica decreases with the proportion of insoluble species and is practically independent of the proportion of soluble species. The porous hydrated layer is rearranged and almost free of soluble species. The ripening of the surface layer makes it protective and inhibits further extraction of the soluble species. These results are in general agreement with the experimental observations on the dissolution of durable glasses.
Similar content being viewed by others
References
R. K. Iler, “The Chemistry of Silica” (Wiley, New York, 1979) Ch. 1.
R. H. Doremus, “Glass Science” 2nd ed. (Wiley, New York, 1994) Ch. 13.
Z. Boksay, G. Bouquet and S. Dobos, Phys. Chem. Glasses 9 (1968) 69.
A. Paul, J. Mater. Sci. 12 (1977) 2246.
L. L. Hench and D. E. Clark, J. Non-Cryst. Solids 28 (1978) 83.
H. Scholze, ibid. 52 (1982) 91.
B. M. J. Smets and T. P. A. Lommen, Phys. Chem. Glasses 23 (1982) 83.
L. A. Chick and L. R. Pederson, Mat. Res. Symp. Proc. 26 (1984) 635.
C. M. Jantzen and M. J. Plodinec, J. Non-Cryst. Solids 67 (1984) 207.
B. Grambow, Mat. Res. Symp. Proc. 44 (1985) 15.
B. E. Sheetz, W. P. Freeborn, D. K. Smith, C. Anderson, M. Zolensky and W. B. White, ibid. 44 (1985) 129.
B. C. Bunker, G. W. Arnold, D. E. Day and P. J. Bray, J. Non-Cryst. Solids 87 (1986) 226.
B. C. Bunker, D. R. Tallant, T. J. Headley, G. L. Turner and R. J. Kirkpatrick, Phys. Chem. Glasses 29 (1988) 106.
H. Scholze, J. Non-Cryst. Solids 102 (1988) 1.
X. Feng, I. L. Pegg, A. Barkatt, P. B. Macedo, S. J. Cucinelli and S. Lai, Nucl. Tech. 85 (1989) 334.
W. L. Bourcier, D. W. Peiffer, K. G. Knauss, K. D. McKeegan and D. K. Smith, Mat. Res. Symp. Proc. 176 (1990) 209.
X. Feng, I. L. Pegg, Y. Guo, A. A. Barkatt and P. C. Macebo, ibid. 176 (1990) 383.
G. Perera and R. H. Doremus, J. Amer. Ceram. Soc. 74 (1991) 1269.
M. Kinoshita, M. Harada, Y. Sato and Y. Hariguchi, ibid. 74 (1991) 783.
T. Advocat, PhD thesis, Université Louis Pasteur, Strasbourg, France, 1991.
T. Advocat, J. L. Crovisier, E. Y. Vernaz, G. Ehret and G. Charpentier, Mat. Res. Soc. Symp. Proc. 212 (1991) 57.
E. Vernaz and J. L. Duchaussoy, Appl. Geochem. 1 (1992) 13.
S. B. Xing, I. S. Muller and I. L. Pegg, Mat. Res. Symp. Proc. 333 (1994) 549.
S. B. Xing, PhD thesis, The Catholic university of America, Washington DC, USA, 1994.
W. L. Ebert and J. J. Mazer, Mat. Res. Symp. Proc. 333 (1994) 27.
S. B. Xing, A. C. Buechele and I. L. Pegg, ibid. 333 (1994) 541.
M. Aertsens and P. Van Iseghem, ibid. 412 (1996) 271.
C. Jegou, PhD thesis Université du Languedoc, Montpellier, France, 1998.
M. Aertsens, Mat. Res. Symp. Proc. 556 (1999) 409.
O. Deruelle, O. Spalla, Ph. Barboux and J. Lambard, J. Non-Cryst. Solids 241 (2000) 237.
C. Jegou, S. Gin and F. LarchÉ, J. Nucl. Mat. 280 (2000) 216.
F. Devreux and M. Kolb, J. Non-Cryst. Solids 242 (1998) 14.
S. B. Santra, B. Sapoval, Ph. Barboux and F. Devreux, C.R. Acad. Sci (Paris) 326 (1998) 129.
P. Meakin, T. Jossang and J. Feder, Phys. Rev. E 48 (1993) 2906.
A. Hernandez-Creus, P. Carro, R. C. Salvarezza and A. J. Arvia, J. Electrochem. Soc. 142 (1995) 3806.
B. Sapoval, S. B. Santra and Ph. Barboux, Europhys. Lett. 41 (1998) 297.
D. Stauffer, “Introduction to Percolation Theory” (Taylor & Francis, London, 1985).
M. Lobanova, L. Maurer, Ph. Barboux, F. Devreux and Y. Minet, Communication to the 24th Symposium on The Scientific Basis for Nuclear Waste Management (Sydney, Australia, 28—31 August 2000), Mat. Res. Symp. Proc, to appear.
J. Hoschen and R. Kopelman, Phys. Rev B 14 (1976) 3428.
B. Grambow, Communication to The International Workshop on Glass in its Disposal Environment (Bruges, Belgium, 11—14 April 2000) J. Nucl. Mat, to appear.
S. Gin and E. Vernaz, Communication to the 24th Symposium on The Scientific Basis for Nuclear Waste Management (Sydney, Australia, 28—31 August 2000), Mat. Res. Symp. Proc, to appear.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devreux, F., Barboux, P., Filoche, M. et al. A simplified model for glass dissolution in water. Journal of Materials Science 36, 1331–1341 (2001). https://doi.org/10.1023/A:1017591100985
Issue Date:
DOI: https://doi.org/10.1023/A:1017591100985