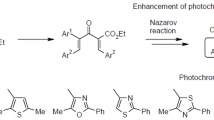
Abstract
There is proposed, and in the case of 2,5-dimethylthiophene carried out, a novel route to the synthesis of 3,4-dithienylfuran-2,5-dione type photochromes. This is done in two stages, the first being a Friedel-Crafts reaction of the starting thiophene with the dichloride of squaric acid and the second is a Baeyer-Villiger oxidation of the 3,4-bis(2,5-dimethyl-3-thienyl)cyclobutenedione to give the target 3,4-bis(2,5-dimethyl-3-thienyl)furan-2,5-dione.
Similar content being viewed by others
REFERENCES
M. M. Krayushkin, S. N. Ivanov, A. Yu. Martynkin, B. V. Lichitskii, A. A. Dudinov, and B. M. Uzhinov, Izv. Akad. Nauk, Ser. Khim., 113 (2001).
P. Ball and L. Garwin, Nature, 355, 761 (1992).
G. M. Tsivgoulis and J.-M. Lehn, Adv. Mater., 9, 39 (1997).
B. L. Feringa, W. F. de Jager, and B. de Lange, Tetrahedron, 49, 8267 (1993).
M. Irie and K. Uchida, Bull. Chem. Soc. Japan, 71, 985 (1998).
M. M. Krayushkin, B. M. Uzhinov, A. Yu. Martynkin, D. L. Dzhavadov, M. A. Kalik, V. L. Ivanov, F. M. Stoyanovich, L. D. Uzhinova, and O. Yu. Zolotarskaya, Int. J. Photoenergy, 1, 183 (1999).
Y. Nakayama, K. Hayashi, and M. Irie, Bull. Chem. Soc. Japan, 64, 789 (1991).
M. Irie, Phosphorus, Sulfur and Silicon, 120/121, 95 (1997).
M. M. Krayushkin, M. A. Kalik, D. L. Dzhavadov and L. G. Vorontsova, Khim. Geterotsikl. Soedin., 929 (1998).
M. M. Krayushkin, M. A. Kalik, D. L. Dzhavadov, A. Yu. Martynkin, A. V. Firsov, and B. M. Uzhinov, Izv. Akad. Nauk, Ser. Khim., 979 (1999).
M. M. Krayushkin, F. M. Stoyanovich, O. Yu. Zolotarskaya, A. Yu. Martynkin, V. L. Ivanov, and B. M. Uzhinov, Izv. Akad. Nauk, Ser. Khim., 1011 (1999).
L. G. Vorontsova, M. M. Krayushkin, Z. A. Starikova, M. A. Kalik, F. M. Stoyanovich, O. Yu. Zolotarskaya, and D. L. Dzhavadov, Izv. Akad. Nauk, Ser. Khim., 74 (2000).
M. M. Krayushkin, M. A. Kalik, D. L. Dzhavadov, L. G. Vorontsova, Z. A. Starikova, A. Yu. Martynkin, V. L. Ivanov, and B. M. Uzhinov, Izv. Akad. Nauk, Ser. Khim., 1778 (2000).
M. M. Krayushkin, F. M. Stoyanovich, O. Yu. Zolotarskaya, I. V. Murav'ev, A. Yu. Martynkin, L. G. Vorontsova, Z. A. Starikova, V. L. Ivanov, and B. M. Uzhinov, Izv. Akad. Nauk, Ser. Khim., 107 (2001).
V. Z. Shirinyan, N. V. Kosterina, A. V. Kolotaev, L. I. Belen'kii, and M. M. Krayushkin, Khim. Geterotsikl. Soedin., 261 (2000).
Jpn. Patent 04.134.061 [92.134.061]; Chem. Abstr., 117, 152774 (1992).
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor, J. Chem. Soc., Perkin Trans. 2, S1 (1987).
A. T. Blomquist and E. A. LaLancette, J. Am. Chem. Soc., 83, 1387 (1961).
M. Ohno, Y. Yamamoto, Y. Shirasaki, and Sh. Eguchi, J. Chem. Soc., Perkin Trans. 1, 263 (1993).
C. B. Hatchard and C. A. Parker, Proc. Roy. Soc., A235, 518 (1956).
E. W. Wagner and A. W. Adamson, J. Am. Chem. Soc., 88, 394 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shirinyan, V.Z., Krayushkin, M.M., Belen'kii, L.I. et al. Photochromic Dihetarylethenes. 8. A Novel Route to the Synthesis of 3,4-Bis(2,5-dimethyl-3-thienyl)furan-2,5-dione as a Potential Photochrome. Chemistry of Heterocyclic Compounds 37, 77–84 (2001). https://doi.org/10.1023/A:1017588717074
Issue Date:
DOI: https://doi.org/10.1023/A:1017588717074