Abstract
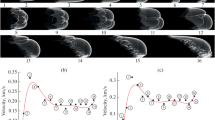
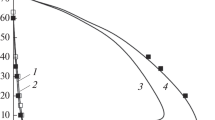
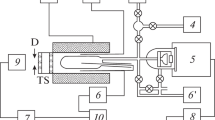
For description of the chemical structure of RDX flames, key reactions and species are selected by numerical solution of the system of equations describing one–dimensional flows of a viscous, heat–conducting, reacting gas at pressures of 0.5—90 atm. The kinetic mechanism consists of 263 elementary steps and 43 species. Literature data on rate constants of elementary steps are considered. Flame structure is calculated for RDX combustion under irradiation. Various reaction paths in the RDX vapor decompositon zone are considered. The effects of the mass flow rate and two–dimensional nature of the gas flow on the flame structure are discussed. Calculation results are compared to experimental data. The structure of various flame zones and the role of individual steps and species in the chemical process are investigated.
Similar content being viewed by others
REFERENCES
N. E. Ermolin and V. E. Zarko, "Mechanism and kinetics of thermal decomposition cyclic nitramines," Fiz. Goreniya Vzryva, 33, No. 3, 10-31 (1997).
N. E. Ermolin and V. E. Zarko, "Modeling of cyclicnitramine combustion," Fiz. Goreniya Vzryva, 34, No. 5, 3-22 (1998).
K. Prasad, R. A. Yetter, and M. D. Smooke, "An eigenvalue method for computing the burning rates of RDX propellants," AIAA Paper No. 96-0880 (1996).
C. F. Melius, "Thermochemical simulation: I and II," in: S. Bulusu (ed.), Chemistry and Physics of Molecular Processes in Energetic Materials, Kluwer, Boston (1990), pp. 21-78.
Y.-C. Liau and V. Yang, "Analysis of RDX monopropellant combustion with two-phase subsurface reactions," J. Propuls. Power, 11, No. 4, 729-739 (1995).
A. Zenin, "HMX and RDX: Combustion mechanism and inuence on modern double-base propellant combustion," ibid., pp. 752-758.
T. P. Parr, D. M. Hanson-Parr, "Solid propellant ame structure," in: T. B. Brill, T. P. Russell, W. C. Tao, et al. (eds.), Decomposition, Combustion and Detonation of Energetic Materials (Symp. held Nov. 27-30, 1995, Boston, U.S.A.), Pittsburgh (1996), pp. 207-219.
C. F. Melius, "The gas-phase ame chemistry of nitramine combustion," in: 25th JANNAF Combustion Meeting, Vol. 2, CPIA 498, 155-162 (1988).
V. M. Mal'tsev and P. F. Pokhil, "Estimate of the heat effect at the initial stage of combustion of propellants and high explosives," Prikl. Mekh. Tekh. Fiz., No. 2, 173-174 (1963).
R. A. Yetter, F. L. Dryer, M. T. Allen, and J. L. Gatto, "Development of gas-phase reaction mechanisms for nitramine combustion," J. Propuls. Power, 11, No. 4, 683-697 (1995).
D. Hanson-Parr and T. Parr, "RDX ame structure," in: Twenty-Fifth Symp. (Int). on Combustion, Combustion Inst., Pittsburgh (1994), pp. 1635-1643.
N. E. Ermolin, O. P. Korobeinichev, L. V. Kuibida, and V. M. Fomin, "Kinetics and mechanism of chemical reactions in RDX ames," Fiz. Goreniya Vzryva, 22, No. 5, 54-64 (1986).
N. E. Ermolin, O. P. Korobeinichev, L. V. Kuibida, and V. M. Fomin, "Analysis of chemical processes in RDX ames," Fiz. Goreniya Vzryva, 24, No. 4, 21-29 (1988).
N. E. Ermolin, O. P. Korobeinichev, L. V. Kuibida, and V. M. Fomin, "Chemical structure and model for RDX ames," in: Structure of Gas-Phase Flames, Proc. Int. Seminar on the Structure of Gas-Phase Flames (Novosibirsk, July 27-31, 1986), Part II, Novosibirsk (1988), pp. 267-279.
J. E. Davidson, M. B. Jeppson, and M. W. Beckstead, "A reduced mechanism for RDX combustion," in: 33rd JANNAF Combustion Meeting (November, 1996) Monterey, CA (1996).
N. E. Ermolin, O. P. Korobeinichev, A. G. Tereshchenko, and V. M. Fomin, "Kinetic calculations and mechanism definition for reactions in an ammonium perchlorate ame," Fiz. Goreniya Vzryva, 18, No. 2, 61-70 (1982).
P. Glarborg, J. A. Miller, and R. J. Kee, "Kinetic modeling and sensitivity analysis of nitrogen oxide formation in well-stirred reactor," Combust. Flame, 65, No. 2, 177-202 (1986).
W. Tsang and R. F. Hampson, "Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds," J. Phys. Chem. Ref. Data, 15, No. 3, 1087-1279 (1986).
R. C. Sausa, W. R. Anderson, D. C. Dayton, et al., "Detailed structure study of a low pressure, stoichiometric H2/N2O/Ar ame," Combust. Flame, 94, No. 4, 407-425 (1993).
C. J. Cobos, H. Hippler, and J. Troe, "High-pressure falloff curves and specific rate constants for the reaction H + O2, HO2, HO + O," J. Phys. Chem., 89, No. 2, 342-349 (1985).
M. W. Slack, "Rate coeficient for H+O2⇔M = HO2⇔M evaluated from shock tube measurements of induction times," Combust. Flame, 28, No. 3, 241-249 (1977).
J. W. Sutherland, P. M. Patterson, and R. Bruce Klemm, "Rate constants for the reaction O(3P)+H2O = OH+OH over the temperature range 1053 K to 2033 K using two direct techniques," in: 23th Symp. (Int). on Combustion, Combustion Inst., Pittsburgh (1990), pp. 51-57.
S. T. Wooldridge, R. K. Hanson, and C. T. Bowman, "Simultaneous laser absorption measurements of CN and OH in a shock tube study of HCN+OH → Products, Int. J. Chem. Kinet., 27, 1075-1087 (1995).
J. A. Miller and C. T. Bowman, "Mechanism and modeling of nitrogen chemistry in combustion," Prog. Energ. Combust. Sci., 15, 287-338 (1989).
P. Glarborg, J. E. Johnsson, and Kim Dam-Johansen, "Kinetics of homogeneous nitrous oxide decomposition," Combust. Flame, 99, No. 3. 523-532 (1994).
W. Tsang, "Chemical kinetic data base for propellant combustion. II. Reactions involving CN, NCO, and HNCO," J. Phys. Chem. Ref. Data, 21, No. 4, 753-791 (1992).
N. S. Wang, D. L. Yang, M. C. Lin, and C. F. Melius, "Kinetics of CN reactions with N2O and CO2," Int. J. Chem. Kinet., 23, No. 2, 151-160 (1991).
T. Ko and A. Fontijn, "High temperature photochemistry kinetics study at the reaction hydrogen atom + nitrogen dioxide → hydroxyl + nitric oxide from 296 to 760 K," J. Phys. Chem., 95, 3984 (1991).
J. D. Mertens, A. Y. Chang, R. K. Hanson, and C. T. Bowman, "A shock tube study of the reactions of NH with NO, O2 and O," Int. J. Chem. Kinet., 23, No. 2, 173-196 (1991).
J. A. Miller and C. F. Melius, "The reactions of imidogen with nitric oxide and molecular oxygen," in: 24th Symp. (Int.) on Combustion, Combustion Inst., Pittsburgh (1992), pp. 719-725.
P. Glarborg, Kim Dam-Johansen, J. A. Miller, et al., "Simulation the thermal DENOx process in ow reactors surface effects and nitrous oxide formation, Int. J. Chem. Kinet., 26, 421-436 (1994).
W. Tsang and J. T. Herron, "Chemical kinetic data base for propellant combustion. I. Reactions involving NO, NO2, HNO, HONO, HCN and N2O," J. Phys. Chem. Ref. Data, 20, No. 4, 609-663 (1991).
R. S. Timonen, E. Ratajczak, and D. Gutman, and A. F. Wagner, "The addition and dissociation reaction H + CO → HCO. 2. Experimental studies and comparison with theory," J. Phys. Chem., 91, No. 20, 5325-5332 (1987).
R. S. Timonen, E. Ratajczak, and D. Gutman, "Kinetics of the reaction between formyl radicals and atomic hydrogen," J. Phys. Chem., 91, No. 3, 692-694 (1987).
R. S. Timonen, E. Ratajczak, and D. Gutman, "Kinetics of the reactions of the formyl radical with oxygen, nitrogen dioxide, chlorine and bromine," J. Phys. Chem., 92, No. 3, 651-655 (1988).
W. C. Gardiner ( Jr.) (ed.), Combustion Chemistry, Springer-Verlag, New York-Berlin-Heidelberg-Tokyo (1984).
M. T. Allen, R. A. Yetter, and F. L. Dryer, "The decomposition of nitrous oxide at 1:5 6 P 6 10:5 atm and 1103 6 T 6 1173 K," Int. J. Chem. Kinet., 27, No. 9, 883-909 (1995).
Y. He and M. C. Lin, "Effects of nitric oxide on the thermal decomposition of methyl nitrate: Overall kinetics and rate constants for the HNO+HNO and HNO+2NO reactions," Int. J. Chem. Kinet., 24, No. 8, 743-760 (1992).
I. R. Thorne and C. F. Melius, "The structure of hydrogen cyanide-nitrogen dioxide premixed ames," in: 23th Symp. (Int.) on Combustion, Combustion Inst., Pittsburgh (1990), pp. 397-403.
R. Atkinson, D. L. Baulch, R. A. Cox, et al., "Evaluated kinetic and photochemical data for atmospheric chemistry: Supplement III," Int. J. Chem. Kinet., 21, 115-150 (1989).
EPA-600/2-76-003, "Survey and Evaluation of Kinetic Data on Reactions in Methane/Air Combustion," (V. S. Engleman) Washington, DC (1976).
Toshimi Takagi, Toshiharu Tatsumi, and Mitsunobu Ogasawara, "Nitric oxide formation from fuel nitrogen in staged combustion: Roles of HCN and NHi," Combust. Flame, 35, 17-25 (1979).
P. Glarborg and J. A. Miller, "Mechanism and modeling of hydrogen cyanide oxidation in a ow reactor," Combust. Flame, 99, Nos. 3/4, 475-483 (1994).
D. Lindackers, M. Burmeister, and P. Roth, "Hightemperature kinetics of the reaction CN + CO2," Combust. Flame, 81, 251-259 (1990).
O. I. Zaslonko, A. M. Tereza, O. N. Kulish, et al., "Kinetic aspects of the reduction in the nitric oxide level in the combustion products using additives of ammonia (De-NOx)," Khim. Fiz., 11, No. 11, 1490-1517 (1992).
P. Roth, M. Y. Louge, and R. K. Hanson, "O-and N-atom measurements in high temperature C2N2 + O kinetics," Combust. Flame, 64, 167-176 (1986).
D. L. Baulch, C. J. Cobos, R. A. Cox, et al., "Evaluated kinetic data for combustion modelling," J. Phys. Chem. Ref. Data, 21, No. 3, 411-638 (1992).
A. Szekely, R. K. Hanson, and C. T. Bowman, "Shock tube study of the reaction between hydrogen cyanide and atomic oxygen," in: 20th Symp. (Int.) on Combustion, Combustion Inst., Pittsburgh (1984), pp. 647-654.
J. A. Miller and C. T. Bowman, "Kinetic modeling of the reduction of nitric oxide in combustion products by isocyanic acid," Int. J. Chem. Kinet., 23, 289-313 (1991).
Y. He, X. Liu, M. C. Lin, and C. F. Melius "Thermal reaction of HNCO with NO2 at moderate temperatures," Int. J. Chem. Kinet., 25, 845-863 (1993).
H. Hippler, J. Troe, and J. Willner, "Shock wave study of the reaction HO2 + HO2 → H2O2 + O2: Confirmation of a rate constant minimum near 700 K," J. Chem. Phys., 93, No. 3, 1755-1760 (1990).
P. Roth and Th. Just, "Kinetics of the high temperature, low concentration CH4 oxidation verified by H and O atom measurements," in: 20th Symp. (Int.) on Combustion, Combustion Inst., Pittsburgh (1984), pp. 807-818.
J. A. Harrison, A. R. Whyte, and L. F. Phillips, "Kinetics of reactions of NH with NO and NO2," Chem. Phys. Let., 129, No. 4, 346-352 (1986).
C.-Y. Lin, H.-T. Wang, M. C. Lin, and C. F. Melius, "A shock tube study of the CH2O + NO2 reaction at high temperatures," Int. J. Chem. Kinet., 22, No. 5, 455-482 (1990).
D. L. Baulch, C. J. Cobos, R. A. Cox, et al., "Summary table of evaluated kinetic data for combustion simulation: Supplement I," Combust. Flame, 98, Nos. 1/2, 59-79 (1994).
R. A. Perry and C. F. Melius, "The rate and mechanism of the reaction of HCN with oxygen atoms over the temperature range 540-900 K," it: 20th Symp. (Int.) on Combustion, Combustion Inst., Pittsburgh (1984), pp. 639-646.
J. D. Mertens, A. Y. Chang, R. K. Hanson, and C. T. Bowman, "Reaction kinetics of NH in the shock tube pyrolysis of HNCO," Int. J. Chem. Kinet., 21, No. 11, 1049-1067 (1989).
L. R. Thorne, M. C. Branch, D. W. Chandler, et al., "Hydrocarbon/nitric oxide interactions in low-pressure ames," in: 21th Symp. (Int.) on Combustion, Combustion Inst., Pittsburgh (1986), pp. 965-977.
P. Glarborg, P. G. Kristensen, S. H. Jensen, and K. Dam-Johansen, "A flow reactor study of HNCO oxidation chemistry," Combust. Flame, 98, No. 3, 241-258 (1994).
G. Stahl and J. Warnatz, "Numerical investigation of time-dependent properties and extinction of strained methane-and propane air amelets," Combust. Flame, 85, No. 3/4, pp. 285-299 (1991).
J. D. Cosqrove and A. J. Owen, "The thermal decomposition of 1,3,5-trinitrohexahydro-1,3,5-triazine (RDX). Part I: The products and physical parameters," Combust. Flame, 22, No. 1, 13-18 (1974).
O. P. Korobeinichev, L. V. Kuibida, et al., "Investigation of ame structure and chemical reactions in ames using mass spectrometry," in: Mass Spectrometry and Chemical Kinetics [in Russian], Nauka, Moscow (1985), pp. 73-93.
T. A. Litzinger, B. L. Fetherolf, Y. Lee, and C. Tang, "Study of the gas-phase chemistry of RDX: Experiment and simulation," J. Propuls. Power, 11, No. 4, 698-703 (1995).
T. B. Brill, "Multiphase chemistry considerations at the burning surface of nitramine monopropellants," ibid., pp. 740-751.
N. E. Ermolin, O. P. Korobeinichev, A. G. Tereshchenko, and V. M. Fomin, "Modeling the kinetics and mechanism of chemical reactions in an ammonium perchlorate ame," Khim. Fiz., No. 2, 1711-1717 (1982).
A. E. Fogelzang, V. P. Sinditskii, et al., "Combustion behavior and ame structure of ammonium dinitramide," in: Combustion and Detonation, Proc. of 28th Int. Annual Conf. of ICT (June 24-July 27, 1997), Karlsruhe, Federal Republic of Germany (1997), pp. 99.1-99.14.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ermolin, N.E., Zarko, V.E. Investigation of the Properties of a Kinetic Mechanism Describing the Chemical Structure of RDX Flames. I. Role of Individual Reactions and Species. Combustion, Explosion, and Shock Waves 37, 123–147 (2001). https://doi.org/10.1023/A:1017563623568
Issue Date:
DOI: https://doi.org/10.1023/A:1017563623568