Abstract
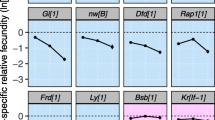
Evolutionary theories of senescence assume that mutations with age-specific effects exist, yet until now, there has been little experimental evidence to support this assumption. In this study, we allowed mutations to accumulate in an outbred, wild population of Drosophila melanogaster to test for age-specific differences in both male mating ability and fecundity. We assayed for age-specific effects of mutations after 10, 20, and 30 generations of mutation accumulation. For mating ability, we found the strongest effects of mutations in the first half of the life span after 20 generations, and at nearly all ages by generation 30. These results are qualitatively consistent with results from a companion study in which age-specific mortality was assayed on the same lines of D. melanogaster. By contrast, effects of fecundity were confined to late ages after 20 generations of mutation accumulation, but by generation 30, as with male mating ability, effects of novel mutations were distributed across all age classes. We discuss several possible explanations for the differences that we observe between generations within traits, and among traits, and the relevance for these patterns to models of aging as well as models of mate choice and sexual selection.
Similar content being viewed by others
References
Beck C.W. & L.A. Powell, 2000. Evolution of female mate choice based on male age: are older males better mates? Evol. Ecol. Res. 2: 107–118.
Blattner, F.R., G. Plunkett, C.A. Bloch, N.T. Perna, V. Burland, M. Riley, J. Collado Vides, J.D. Glasner, C.K. Rode, G.F. Mayhew, J. Gregor, N.W. Davis, H.A. Kirkpatrick, M.A. Goeden, D.J. Rose, B. Mau & Y. Shao, 1997. The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1474.
Charlesworth, B., 1990. Optimization models, quantitative genetics, and mutation. Evolution 44: 520–538.
Crow, J.F. & M. Kimura, 1970. An Introduction to Population Genetics Theory. Harper and Row Publishers, New York.
Drake, J.W., 1991. A constant rate of spontaneous mutation in DNAbased microbes. Proc. Natl. Acad. Sci. USA 88: 7160–7164.
Fry, J.D., S.L. Heinsohn & T.F.C. Mackay, 1996. The contribution of new mutations to genotype-environment interaction for fitness in Drosophila melanogaster. Evolution 50: 2316–2327.
Fry, J.D., P.D. Keightley, S.L. Heinsohn & S.V. Nuzhdin, 1999. New estimates of the rates and effects of mildly deleterious mutation in Drosophila melanogaster. P. Natl. Acad. Sci. USA 96: 574–579.
Garcia-Dorado, A., J.L. Monedero & C. Lopez-Fanjul, 1998. The mutation rate and the distribution of mutational effects of viability and fitness in Drosophila melanogaster. Genetica 103: 255–265.
Goffeau, A., B.G. Barrell, H. Bussey, R.W. Davis, B. Dujon, H. Feldmann, F. Galibert, J.D. Hoheisel, C. Jacq, M. Johnston, E.J. Louis, H.W. Mewes, Y. Murakami, P. Philippsen, H. Tettelin & S.G. Oliver, 1996. Life with 6000 genes. Science 274: 546, 563–567.
Hamilton, W.D., 1966. The moulding of senescence by natural selection. J. Theor. Biol. 12: 12–45.
Hansen, T.F. & D.K. Price, 1995. Good genes and old age: Do old mates provide superior genes? J. Evol. Biol. 8: 759–778.
Helfand, S.L., K.J. Blake & B. Rogina, 1995. Temporal patterns of gene expression in the antenna of the adult Drosophila melanogaster. Genetics 140: 549–555.
Holm, S., 1979. A simple sequential rejective multiple test procedure. Scand. J. Stat. 6: 65–70.
Hughes, K.A., 1995a. The evolutionary genetics of male lifehistory characters in Drosophila melanogaster. Evolution 49: 521–537.
Hughes, K.A., 1995b. The inbreeding decline and average dominance of genes affecting male life-history characters in Drosophila melanogaster. Genet. Res. 65: 41–52.
Hughes, K.A. & B. Charlesworth, 1994. A genetic analysis of senescence in Drosophila. Nature 367: 64–66.
Iwasa, 6Y., A. Pomiankowski & S. Nee, 1991. The evolution of costly mate preferences II. The ‘handicap’ principle. Evolution 45: 1431–1442.
Jacobs, K.L. & D.W. Grogan, 1997. Rates of spontaneous mutation in an archaeon from geothermal environments. J. Bacteriol. 179: 3298–3303.
Keightley, P.D., 1994. The distribution of mutation effects on viability in Drosophila melanogaster. Genetics 138: 1315–1322.
Keightley, P.D., 1996. Nature of deleterious mutation load in Drosophila. Genetics 144: 1993–1999.
Keightley, P.D., 1998. Inference of genome-wide mutation rates and distributions of mutation effects for fitness traits: A simulation study. Genetics 150: 1283–1293.
Keightley, P.D. & A. Caballero, 1997. Genomic mutation rates for lifetime reproductive output and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 94: 3823–3827.
Kibota, T.T. & M. Lynch, 1996. Estimate of the genomic mutation rate deleterious to overall fitness in E-coli. Nature 381: 694–696.
Kokko, H. & J. Lindström, 1996. Evolution of female preference for old mates. Proc. R. Soc. Lond. B. 263: 1533–1538.
Kondrashov, A.S., 1994. Sex and deleterious mutation. Nature 369: 99–100.
Kondrashov, A.S., 1988. Deleterious mutations and the evolution of sexual reproduction. Nature 336: 435–440.
Kosuda, K., 1985. The aging effect on male mating activity in Drosophila melanogaster. Behav. Genet. 15: 297–303.
Lande, R., 1994. Risk of population extinction from fixation of new deleterious mutations. Evolution 48: 1460–1469.
Lansing, A.I., 1947. A transmissible, cumulative, and reversible factor in aging. J. Gerontol. 2: 228–239.
LeClerc, J.E., B.G. Li, W.L. Payne & T.A. Cebula, 1996. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274: 1208–1211.
Lynch, M., J. Blanchard, D. Houle, T. Kibota, S. Schultz, L. Vassilieva & J. Willis, 1999. Perspective: Spontaneous deleterious mutation. Evolution 53: 645–663.
Lynch, M., R. Burger, D. Butcher & W. Gabriel, 1993. The mutational meltdown in asexual populations. J. Hered. 84: 339–344.
Lynch, M., J. Conery & R. Burger, 1995a. Mutation accumulation and the extinction of small populations. Am. Natur. 146: 489–518.
Lynch, M., J. Conery & R. Burger, 1995b. Mutational meltdowns in sexual populations. Evolution 49: 1067–1080.
Manly, B.F.J., 1997. Randomization, Bootstrap and Monte Carlo Methods in Biology. Chapman and Hall, New York.
Matic, I., M. Radman, F. Taddei, B. Picard, C. Doit, E. Bingen, E. Denamur & J. Elion, 1997. Highly variable mutation rates in commensal and pathogenic Escherichia coli. Science 277: 1833–1834.
Medawar, P.B., 1946. Old age and natural death. Modern Quart. 2: 30–49.
Medawar, P.B., 1952. An Unsolved Problem in Biology. H.K. Lewis, London.
Mukai, T., 1964. The genetic structure of natural populations of Drosophila melanogaster. I. Spontaneous mutation rate of polygenes controlling viability. Genetics 50: 1–19.
Mukai, T., S.I. Chigusa, L.E. Mettler & J.F. Crow, 1972. Mutation rate and dominance of genes affecting viability in Drosophila melanogaster. Genetics 72: 335–355.
Nuzhdin, S.V., E.G. Pasyukova, C.L. Dilda, Z.-B. Zeng & T.F.C. Mackay, 1997. Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 94: 9734–9739.
Ohnishi, O., 1977. Spontaneous and ethyl methanesulfonateinduced mutations controling viability in Drosophila melangoster. II. Homozygous effects of polygenic mtuations. Genetics 87: 529–545.
Partridge, L., A. Green & K. Fowler, 1987. Effects of eggproduction and of exposure to males on female survival in Drosophila melanogaster. J. Insect Physiol. 33: 745–749.
Pletcher, S.D., D. Houle & J.W. Curtsinger, 1998. Age-specific properties of spontaneous mutations affecting mortality in Drosophila melanogaster. Genetics 148: 287–303.
Pomiankowski, A., Y. Iwasa & S. Nee, 1991. The evolution of costly mate preferences. I. Fisher and biased mutation. Evolution 45: 1422–1430.
Promislow, D.E.L., M. Tatar, A. Khazaeli & J.W. Curtsinger, 1996. Age-specific patterns of genetic variance in Drosophila melanogaster. I. Mortality. Genetics 143: 839–848.
Rogina, B. & S.L. Helfand, 1995. Regulation of gene-expression is linked to life-span in adult Drosophila. Genetics 141: 1043–1048.
Rogina, B. & S.L. Helfand, 1996. Timing of expression of a gene in the adult Drosophila is regulated by mechanisms independent of temperature and metabolic rate. Genetics 143: 1643–1651.
Rose, M. & B. Charlesworth, 1981a. Genetics of life-history evolution in Drosophila melanogaster. II. Exploratory selection experiments. Genetics 97: 187–196.
Rose, M.R. & B. Charlesworth, 1981b. Genetics of life-history evolution in Drosophila melanogaster. I. Sib analysis of adult females. Genetics 97: 173–186.
Schultz, S.T., M. Lynch & J.H. Willis, 1999. Spontaneous deleterious mutation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96: 11393–11398.
Shabalina, S.A., L.Y. Yampolsky & A.S. Kondrashov, 1997. Rapid decline of fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Natl. Acad. Sci. USA 94: 13034–13039.
Shaw, F.H., D.E. Promislow, M. Tatar, K.A. Hughes & C.J. Geyer, 1999. Toward reconciling inferences concerning genetic variation in senescence in Drosophila melanogaster. Genetics 152: 553–566.
Tatar, M., D.E.L. Promislow, A. Khazaeli & J. Curtsinger, 1996. Age-specific patterns of genetic variance in Drosophila melanogaster: II. Fecundity and its genetic correlation with age-specific mortality. Genetics 143: 849–858.
Tatar, M., S. Chien & N.K. Priest. In press. Negligible senescence during reproductive diapause in Drosophila melanogaster. Am. Natur.
Vassilieva, L.L. & M. Lynch, 1999. The rate of spontaneous mutation for life-history traits in Caenorhabditis elegans. Genetics 151: 119–129.
Vaupel, J.W. & A.I. Yashin, 1985. Heterogeneity's ruses: Some surprising effects of selection on population dynamics. Am. Statist. 39: 176–195.
Williams, K.D. & M.B. Sokolowski, 1993. Diapause in Drosophila melanogaster females: a genetic analysis. Heredity 71: 312–317.
Willis, J.H., 1999. Inbreeding load, average dominance and the mutation rate for mildly deleterious alleles in Mimulus guttatus. Genetics 153: 1885–1898.
Yampolsky, L.Y., L. Pearse & D.E.L. Promislow, 2001. Age-specific effects of novel mutations in Drosophila: I. Mortality rates. Genetica 110: 11–29.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mack, P.D., Lester, V.K. & Promislow, D.E.L. Age-specific Effects of Novel Mutations in Drosophila Melanogaster II. Fecundity and Male Mating Ability. Genetica 110, 31–41 (2000). https://doi.org/10.1023/A:1017538505627
Issue Date:
DOI: https://doi.org/10.1023/A:1017538505627