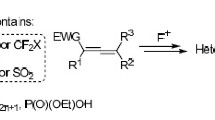
Abstract
Published data on the synthesis of various heterocyclic compounds by the reaction of nucleophiles with electrophilic olefins containing a nucleophilic group are reviewed and analyzed.
Similar content being viewed by others
REFERENCES
Z. Rappoport, Adv. Phys. Org. Chem., 7, 1 (1969).
G. Modena, Acc. Chem. Res., 4, 73 (1971).
S. Patai (Editor), Chemistry of Alkenes [Russian translation], Khimiya, Leningrad (1969), p. 302.
P. Stang, Z. Rappoport, J. Hanak, and K. Subramian, Vinyl Cations, Chap. 5, Academic Press, New York (1979).
P. Stang, Acc. Chem. Res., 11, 107 (1978).
M. I. Rybinskaya, A. N. Nesmeyanov, and N. K. Kochetkov, Usp. Khim., 38, 961 (1969).
Z. Rappoport, Acc. Chem. Res., 14, 7 (1981).
Y. A. Ammar, A. M. Sh. El-Sharief, M. A. Zahran, M. Z. El-Said, and U. H. El-Said, J. Chem. Res. Synop., No. 3, 324 (1995).
W. Ried and S. Aboul-Fetouh, Tetrahedron, 44, 7155 (1988).
J. N. Vishvakarma, B. K. R. Choldhury, H. Iva, and H. Junjappa, Indian J. Chem., 24, 472 (1985).
V. J. Ram and N. Haque, Indian J. Chem. B, 34, 521 (1995).
M. Taniguni, I. Ivadzava, and K. Nissan, Jpn. Patent Appl. 58-140074; Ref. Zh. Khim., 2O 394 P (1985).
W. Holzer and E. Schmid, J. Heterocycl. Chem., 32, 1341 (1995).
K. Nagarajan, V. P. Arya, and S. J. Shenoy, J. Chem. Res. Synop., No. 5 (1968); J. Chem. Res. Microfiche, No. 13–17, 166; Ref. Zh. Khim., 1 Zh 250 (1987).
V. A. Dorokhov, A. V. Komkov, and B. I. Ugrak, Izv. Ross. Akad. Nauk. Ser. Khim., 1429 (1993).
P. Schmidt, J. Druey, Helv. Chim. Acta, 39, 986 (1956).
P. Giori, A. S. Verones, C. V. Vicentim, and M. J. Guarnerri, J. Heterocycl. Chem., 22, 1093 (1985).
M. Jochheim, H. G. Krug, R. Neidlein, and C. Krieger, Heterocycles, 41, 1235 (1995).
R. Bartroli, L. Lami, M. Diaz, J. Quincoces, and K. Peseke, Rev. ICIDCA, 22, 35 (1988); Ref. Zh. Khim., 19 Zh 181 (1989).
R. J. Quinn, P. J. Scammells, C. H. L. Kennard, and G. Smith, Aust. J. Chem., 44, 1795(1991).
S. G. Krivokolysko, Yu. A. Sharanin, and V. D. Dyachenko, Zh. Obshch. Khim., 65, 878 (1995).
G. Doleschall and P. Seres, J. Chem. Soc. Perkin Trans. 1, 1875 (1988).
W. Spiegler and N. Gotz, Synthesis, 69 (1986).
E. Dominiguez, E. Ibes, E. Martinez de Marigorta, J. K. Palacios, and R. SanMartin, J. Org. Chem., 61, 5435 (1996).
Z. N. Huang and Z. M. Li, Chem. J. Chin. Univ., 16, 1740 (1995); Ref. Zh. Khim., 1 Zh 260 (1997).
A. Alberola, L. F. Antolin, A. M. Gonzalez, M. A. Laguna, and F. J. Pulido, Heterocycles, 25 (1987); Spec. Issue, p. 393.
E. De la Cuesta and C. Avendano, J. Heterocycl. Chem., 22, 337 (1985).
A. M. Badawey, El-Sayed, and T. Kappe, Arch. Pharm., 330, 59 (1997).
A. Bajnati, M. Habert, K. Takagi, and H. Terada, Heterocycles, 29, 1583 (1989).
K. Takagi, A. Bajnati, and M. Habert, J. Heterocycl. Chem., 27, 1847 (1990).
G. B. Barlin, B. Kotecka, and K.H. Rieckmann, Aust. J. Chem., 43, 647 (1996).
J. M. Delacotte, H. Parrot-Lopez, J. Renault, S. Cros, and C. Paoletti, Eur. J. Med. Chem., 22, 187 (1987).
V. J. Ram, N. Haque, and M. Nath, Indian J. Chem., 32, 754 (1993); Ref. Zh. Khim., 6 Zh 196 (1995).
M. Peshmukh, M. Mittelbach, and H. Junek, Monatsh. Chem., 126, 91 (1995).
H.-W. Schmidt, G. Koitz, and H. Junek, J. Heterocycl. Chem., 24, 1305 (1987).
D. C. Agathocieous, H. H. Moharram, and G. Shaw, J. Chem. Res., 374 (1990).
K. Grohe, H. J. Zeiler, and K. Metzger, Germ. Patent Appl. 3326190; Ref. Zh. Khim., 1 O 135 P (1986).
T. V. Ivanyuk, A. V. Kadushkin, N. P. Solov'eva, and V. G. Granik, Khim.-Farm. Zh., 30,No. 6, 47 (1996).
Z. T. Huang and M. X. Wang, Synth. Commun., 21, 1177 (1991).
M. T. Cocco, C. Congiu, A. Maccioni, and A. Plumitallo, J. Heterocycl. Chem., 26, 1859 (1989).
M. T. Cocco, C. Congiu, and A. Maccioni, J. Heterocycl. Chem., 27, 1143 (1990).
A. A. Krauze, E. E. Liepin'sh, Yu. E. Pelcher, Z. A. Kalme, I. V. Dipan, and G. Ya. Dubur, Khim. Geterotsikl. Soedin., 95 (1985).
B. Erdmann, A. Knoll, and J. Liebscher, J. Prakt. Chem., 330, 1015 (1988).
I. A. Aliev, A. I. Mikhaleva, S. Kh. Bairamova, E. I. Akhmedov, Zh. Org. Khim., 22, 489 (1986).
M. Brimble and A. D. Johnston, Tetrahedron, 50, 4887 (1994).
K. Sakano, S. Yokohama, I. Haykawa, S. Atarashi, and S. Kadoya, Agric. Biol. Chem., 51, 1265 (1987).
R. M. Stern, French Patent Appl. 2544316; Ref. Zh. Khim., 14 O 146P (1985).
U. Jordis, F. Sauter, M. Rudolf, and G. Cai, Monatsh. Chem., 119, 761 (1988).
L. E. Baugh, S. Sunder, and J. E. Lewis, J. Med. Chem., 28, 298 (1985).
T. S. T. Wang, R. A. Dawwaz, and R. L. van Heertum, J. Labelled Compounds and Radiopharm., 36, 314 (1995); Ref. Zh. Khim., 1 Zh 223 (1996).
C. A. Leach, T. H. Brown, R. J. Ife, D. J. Keeling, M. E. Parsons, C. J. Theobald, and K. J. Wiggal, J. Med. Chem., 38, 2748 (1995).
J. Jurgens, H. Schedletzky, P. Heising, J. L. Seydel, B. Wiedemann, and U. Holzgrabe, Arch. Pharm., 329, 179 (1996).
K. Kosegi, D. Savada, and S. Moristita, Jpn. Patent Appl. 61-106557; Ref. Zh. Khim., 11 O 124 P (1987).
T. P. Culbertson, J. M. Domagala, P. Peterson, S. Bongers, and J. B. Nichols, J. Heterocycl. Chem., 24, 1509 (1987).
S. Simidzu, F. Yagibsi, H. Takano, Ya. Sankei, and K. Yugen, Jpn. Patent Appl. 62-263157; Ref. Zh. Khim., 2 O 73 P (1989).
R. G. Glushkov, E. V. Adamskaya, A. F. Oleinik, V. A. Silin, and E. N. Padeiskaya, Khim.-Farm. Zh., 20, 313 (1986).
M. Artico, F. Corelli, S. Massa, G. Stefancich, S. Panico, and N. Simonetti, Farmaco. Ed. Sci., 39, 910 (1984).
G. Leclerc, G. Marciniak, N. Defcker, and J. Schwartz, Bioorg. Heterocycles (1986); Synth., Mech. and Bioactiv., Proc. 4th FECHEM Conf., Houthelen, May 25–28 (1986), Amsterdam (1996).
V. Awashi and S. M. S. Chauhan, Indian J. Chem., 30, 352 (1991).
U. Jordis, Monatsh. Chem., 117, 1339 (1986).
N. Abe, Bull. Chem. Soc. Japan, 64, 2393 (1991).
Xu Caiding, Wu Luling, Y. Zheng, and X. Huang, Huaxue Shiji. Chem. Reagents, 353 (1996); Ref. Zh. Khim., 6 Zh 167 (1998).
Y. Tominaga, T. Michioka, K. Moryama, and A. Hosomi, J. Heterocycl. Chem., 125, 1217 (1990).
A. K. El-Shafei, A. M. Soliman, A. A. R. Sulfan, and A. M. M. El-Sagher, Gazz. Chim. Ital., 3, 115 (1995).
G. B. Barlin and W. L. Tan, Aust. J. Chem., 37, 1065 (1984).
F. Pochat, Tetrahedron, 42, 3537 (1986).
R. F. Collins, P. Knowles, L. C. Saunders, F. J. Tierney, and P. J. Warne, British Patent Appl. 2136429; Ref. Zh. Khim., 9 O 90 P (1985).
A. Marcos, C. Pedegral, and C. Avendano, Tetrahedron, 51, 1763 (1995).
E. Pinto de Souza and P. S. Fernandes, Indian J. Chem., 33, 795 (1994).
J. A. Garcia, A. Sanchez, and M. Nogueras, J. Heterocycl. Chem., 26, 1089 (1989).
G. L. Anderson and S. G. Richardson, J. Heterocycl. Chem., 22, 1735 (1985).
P. Molina, A. Arques, I. Cartagena, M. A. Alias, F. F. M. Concepcion, and F. H. Cano, Lieb. Ann. Chem., No. 2, 133 (1988).
H. Nakazumi, T. Endo, T. Nakaue, and T. Kitao, J. Heterocycl. Chem., 22, 89 (1985).
M. S. R. Murhy, Y. V. D. Nageswar, N. A. V. Reddy, T. Ramalingam, and P. B. Suttur, J. Heterocycl. Chem., 26, 473 (1989).
C. Jolivet, C. Rivalle, and E. Bisagni, Heterocycles, 43, 995 (1996).
S. Chimichi, B. Cosimelli, F. Bruni, and A. Costanozo, J. Chem. Soc. Perkin Trans. 2, 209 (1993).
M. B. El-Ashmawy, I. A. Shehata, H. I. El-Subbagh, and A. A. El-Emam, Gazz. Chim. Ital., 121, 113 (1991).
D. M. Osimov, S. Sh. Shukurov, I. M. Nasyrov, K. S. Zakharov, and R. A. Karakhanov, 18th Conf. on the Chemistry and Technology of Organic Compounds of Sulfur. Abstracts [in Russian], Part 2, Kazan' (1992), p. 124.
G. H. Elgemeie, H. A. Ali, and A. M. Elzanate, J. Chem. Res. Synop., No. 7, 340 (1996).
G. M. Abdala, Sr. Sowell, and J. Walter, J. Heterocycl. Chem., 27, 1201 (1990).
D. Ilavsky, V. Bobosik and A. Martvcn, Chem. Pap. (CSSR), No. 4, 527 (1985); Ref. Zh. Khim., 7 Zh 336 (1986).
S. M. Sherif and A. M. Hussein, Monatsh. Chem., 128, 687 (1997).
K. Heleyova and D. Havsky, Monatsh. Chem., 126, 1359 (1995).
V. Moinet-Hedin, T. Tabka, P. Gauduchon, J. Y. le Talaer, B. Letois, J. C. Lancelot, S. Rault, and M. Robba, Eur. J. Med. Chem., 28, 721 (1993).
Y. Tominaga, Y. Ichichara, T. Mori, C. Kamio, and A. Hosomi, J. Heterocycl. Chem., 27, 263 (1990).
A. M. Shestopalov, V. P. Litvinov, Yu. A. Sharanin, I. A. Aitov, and L. A. Rodinovskaya, Izv. Akad. Nauk SSSR. Ser. Khim., 939 (1991).
A. Kakehi, S. Ito, and K. Matsubara, Bull. Chem. Soc. Japan, 68, 2409 (1995).
A. Kaleh, S. Ito, K. Watanabe, T. Ono, and T. Miyazima, J. Chem. Res. Synop., No. 26, 18 (1980).
A. Kakehi, S. Ito, T. Ohizumi, and T. Maeda, J. Org. Chem., 47, 369 (1982).
Y. Tominaga, Y. Shiroshita, T. Kurokawa, H. Gotou, Y. Matsuda, and A. Hosomi, J. Heterocycl. Chem., 26, 477 (1989).
Y. Matsuda, H. Gotou, K. Katou, H. Matsumoto, M. Yamashita, K. Takahashi, and S. Ide, Heterocycles, 31, 977 (1990).
Y. Matsuda, Y. Chiyomaru, C. Motokawa, and T. Nishiyori, Heterocycles, 41, 329 (1995).
A. Kakehi, S. Ito, and T. Yotsuya, Chem. Pharm. Bull., 34, 2435 (1986).
I. A. Aitov and Yu. A. Sharanin, Zh. Org. Khim., 2, 291 (1994).
Ya. Yu. Yakunin and I. A. Aitov, XVII Ukrainian Conference on Organic Chemistry. Abstracts [in Russian], Part 1, Khar'kov (1995), p. 158.
V. P. Litvinov, L. A. Rodinovskaya, Yu. A. Sharanin, A. M. Shestopalov, and A. Sening, Sulfur Rep., 13, 1 (1992).
V. P. Litvinov, S. G. Krivokolysko, and V. D. Dyachenko, Khim. Geterotsikl. Soedin., 579 (1999).
W. D. Jones, R. C. Dage, and R. A. Schnettler, US P atent 4568751; Ref. Zh. Khim., 17 O 71 P (1986).
G. Y. Lesher and B. Singh, US Patent 4517190; Ref. Zh. Khim., 1 O 127 P (1986).
G. Y. Lesher and B. Singh, US Patent 4595762; Ref. Zh. Khim., 5 O 104 P (1987).
W. D. Jones, R. A. Schnettler, and R. C. Dage, US Patent 4732982; Ref. Zh. Khim., 5 O 47 P (1989).
W. D. Jones, R. A. Schnettler, and R. C. Dage, US Patent 4731371; Ref. Zh. Khim., 1 O 67 P (1989).
W. D. Jones, R. C. Dage, and R. A. Schnettler, US Patent 4769380; Ref. Zh. Khim., 12 O 89 P (1989).
S. Nikagava, H. Fukatsu, I. Kato, A. Murasé, and N. Harada, Jpn. Patent Appl. 61-267588; Ref. Zh. Khim., 4 O 106 P (1988).
I. Mosbi, P. Shenone, and G. Menozzi, J. Heterocycl. Chem., 22, 1503 (1985).
D. M. Stout, D. M. Gamamoto, and G. C. Barcelon, US Patent 4555517; Ref. Zh. Khim., 13 O 89 P (1986).
S. Nikagava, H. Fukatsu, I. Kato, A. Murasé, N. Harada, and S. K. K. Ban'yu, Jpn. Patent Appl. 61-140583; Ref. Zh. Khim., 15 O 93 P (1987).
D. M. Stout, D. M. Gamamoto, and G. C. Barcelon, US Patent 4657919; Ref. Zh. Khim., 1 O 54 P (1988).
G. Y. Lesher and B. Singh, US Patent 4539327; Ref. Zh. Khim., 8 O 105 P (1986).
T. L. Su, J. T. Huang, T. C. Chon, G. M. Otter, F. M. Sirotnak, and K. A. Watanabe, J. Med. Chem., 31, 1209 (1988).
L. Mosti, P. Schenone, M. M. M. Del, P. Dorigo, D. Fraccarollo, G. Santostasi, M. D'Amico, M. Falciani, and E. Lampa, Farmaco, 49, 559 (1994).
W. C. Faith, H. F. Campbell, and D. E. Kubia, US Patent 4743608; Ref. Zh. Khim., 9 O 57 P (1989).
G. Y. Lesher, C. J. Opalka, and D. F. Page, US Patent 4599423; Ref. Zh. Khim., 9 O 109 P (1987).
I. A. Bristol and J. Sircar, US Patent 4607030; Ref. Zh. Khim., 8 O 118 P (1987).
G. Y. Lesher, US Patent 4517192; Ref. Zh. Khim., 1 O 83 P (1986).
I. A. Bristol and J. Sircar, US Patent 4503061; Ref. Zh. Khim., 20 O 97 P (1985).
D. R. Duncan, D. Johnson, and R. S. Andrews, J. Labelled Compounds and Radiopharm., 22, 197 (1985); Ref. Zh. Khim., 18 Zh 225 (1985).
H. Fukatsu, I. Kato, S. Murasé, N. Harada, S. Nakagava, and S. Ban'yu, Jpn. Patent Appl. 61-243080; Ref. Zh. Khim., 23 O 99 P (1987).
M. Yamanaka, K. Milké, S. Suda, H. Ohara, and T. Ogawa, Jpn. Patent Appl. 61-218589; Ref. Zh. Khim., 4 O 98 P (1988).
W. Stelzer, E. Hofferber, and A. G. Beiersdorf, Germ. Patent Appl. 3424685; Ref. Zh. Khim., 23 O 111 P (1986).
A. Rumler, S. Heer, A. Hagen, H.-J. Heidrich, H.-J. Janseh, B. Gentsch, and H. Anderle, GDR Patent 252372; Ref. Zh. Khim., 15 O 69 P (1988).
K. O. Gelotte, US Patent 4469871; Ref. Zh. Khim., 14 O 104 P (1985).
G. E. H. Elgemeie and R. M. M. Mahfouz, Phosph., Sulfur, Silicon, Relat. Elem., 46, 95 (1989).
F. A. Abu-Shanab, A. D. Redhouse, J. R. Thompson and B. J. Wakefield, Synthesis, No. 5, 557 (1995).
H. F. Campbell and H. William, US Patent 4514400; Ref. Zh. Khim., 1 O 81 P (1986).
G. L. Lesher, C. J. Opalka, and D. F. Page, US Patent 4559352; Ref. Zh. Khim., 16 O 74 P (1986).
H. F. Campbell, F. Henry, and H. William, US Patent 4539321; Ref. Zh. Khim., 9 O 100 P (1986).
H. F. Campbell and H. William, US Patent 4593028; Ref. Zh. Khim., 3 O 103 P (1987).
G. L. Lesher, C. J. Opalka, D. F. Page, and R. M. McGarry, US Patent 4465686; Ref. Zh. Khim., 14 O 106 P (1985).
G. L. Lesher, C. J. Opalka, and D. F. Page, US Patent 4515797; Ref. Zh. Khim., 24 O 125 P (1985).
E. C. Taylor, G. S. K. Wong, S. R. Fletcher, P. J. Harrington, G. P. Beardsley, and C. J. Shih, Symposium, Montreal, June 15–20 (1986); Ref. Zh. Khim., 3 E 146 (1988).
E. E. Apenova, Yu. A. Sharanin, B. M. Zolotarev, and V. P. Litvinov, Izv. Akad. Nauk SSSR. Ser. Khim., 406 (1986).
V. P. Litvinov, E. E. Apenova, Yu. A. Sharanin, and A. M. Shestopalov, Zh. Org. Khim., 21, 669 (1985).
V. P. Litvinov, E. E. Apenova, Yu. A. Sharanin, V. N. Nesterov, V. E. Shklover, and Yu. T. Struchkov, Izv. Akad. Nauk SSSR. Ser. Khim., 145 (1986).
V. P. Litvinov, E. E. Apenova, and Yu. A. Sharanin, Izv. Akad. Nauk SSSR. Ser. Khim., 386 (1987).
V. P. Litvinov, E. E. Apenova, Yu. A. Sharanin, and A. M. Shestopalov, Izv. Akad. Nauk SSSR. Ser. Khim., 2408 (1984).
Yu. A. Sharanin, V. P. Litvinov, V. Yu. Mortikov, and V. N. Nesterov, Zh. Obshch. Khim., 57, 959 (1987).
Yu. A. Sharanin, A. M. Shestopalov, V. P. Litvinov, G. V. Klokol, V. Yu. Mortikov, and A. S. Demerkov, Zh. Org. Khim., 24, 854 (1988).
L. A. Rodinovskaya, V. A. Promonenkov, Yu. A. Sharanin, V. I. Shvedov, A. M. Shestopalov, V. P. Litvinov, and V. Yu. Mortikov, Zh. Org. Khim., 21, 1578 (1985).
L. A. Rodinovskaya, A. M. Shestopalov, V. K. Promonenkov, and V. P. Litvinov, Zh. Org. Khim., 22, 223 (1986).
V. N. Nesterov, L. A. Rodinovskaya, V. P. Litvinov, Yu. A. Sharanin, A. M. Shestopalov, V. Yu. Mortikov, V. I. Shvedov, V. E. Shklover, and Yu. T. Struchkov, Izv. Akad. Nauk SSSR. Ser. Khim., 140 (1988).
V. K. Promonenkov, A. M. Shestopalov, Yu. A. Sharanin, V. P. Litvinov, L. A. Rodinovskaya, B. M. Zolotarev, V. D. Sokovykh, B. V. Rozynov, and Ya. Ya. Krymskii, Zh. Org. Khim., 21, 1963 (1985).
A. M. Shestopalov, L. A. Rodinovskaya, Yu. A. Sharanin, and V. P. Litvinov, Zh. Org. Khim., 58, 840 (1988).
V. P. Litvinov, Yu. A. Sharanin, V. K. Promonenkov, L. A. Rodinovskaya, A. M. Shestopalov, and V. Yu. Mortikov, Izv. Akad. Nauk SSSR. Ser. Khim., 1869 (1984).
V. P. Litvinov, Yu. A. Sharanin, L. A. Rodinovskaya, A. M. Shestopalov, V. Yu. Mortikov, and V. K. Promonenkov, Izv. Akad. Nauk SSSR. Ser. Khim., 2760 (1984).
L. A. Rodinovskaya, Yu. A. Sharanin, V. P. Litvinov, A. M. Shestopalov, V. K. Promonenkov, B. M. Zolotarev, and V. Yu. Mortikov, Zh. Org. Khim., 21, 2439 (1985).
L. A. Rodinovskaya, Yu. A. Sharanin, A. M. Shestopalov, and V. P. Litvinov, Khim. Geterotsikl. Soedin., 805 (1988).
H. Nakagawa, N. Ando, N. Harada, and S. Ban'yu, Jpn. Patent Appl. 60-94964; Ref. Zh. Khim., 7 O 75 P (1986).
Y. Tominaga, A. Ushirogochi, and Y. Matsuda, J. Heterocycl. Chem., 24, 1557 (1987).
Y. Tominaga, M. Kawabe, and A. Hosomi, J. Heterocycl. Chem., 24, 1325 (1987).
F. Al-Omran, N. Al-Awadhi, K. M. M. Abdel, K. Kaul, A. A. El-Khair, and M. H. Elnagdi, J. Chem. Res. Synop., No. 3, 84 (1997).
K. Peseke, R. M. Bartroli, and S. J. Quincoces, Wiss. Z. Wilhelm-Pieck-Univ., Rostok. Naturwiss, 37, 46 (1988); Ref. Zh. Khim., 7 Zh 198 (1991).
W. A. Boulanger and J. A. Katzenellenbogen, J. Med. Chem., 29, 1159 (1986).
A. B. Zolotoi, P. M. Lukin, A. I. Prokhorov, V. M. Romanov, O. E. Nasakin, L. O. Atovmyan, and V. A. Bakhmisov, Dokl. Akad. Nauk, 311, 122 (1990).
E. O. Hermi Osmo and A. M. Paakkanen, J. Org. Chem., 52, 5275 (1987).
H. W. Schmidt and M. Klade, Lieb. Ann. Chem., 3, 257 (1988).
M. Sarkar, S. Chatlopadhyay, and K. Mahalanabis, Indian J. Chem., 25, 1133 (1986).
B. Potthoff and E. Breitmainer, Synthesis, No. 7, 584 (1986).
M. Augustin, J. Frank, and M. Kohler, J. Pr. Chem., 326, 594 (1984).
Y. Matsuda, H. Gotou, K. Katou, and H. Matsumoto, Chem. Pharm. Bull., 37, 1188 (1989).
I. Hermecz, Z. Mesraros, K. Simon, L. Szabo, and Z. Pal, J. Chem. Soc. Perkin Trans. 1, 1795 (1984).
Kh. Psikava, T. Uno, Kh. Miyamoto, K. Nakagawa, and S. Otsuka, Jpn. Patent Appl. 57-186287; Ref. Zh. Khim., 13 O 132 P (1985).
S. Kambe, K. Saito, T. Oki, A. Sakirai, and H. Madorikawa, Synthesis, No. 7, 601 (1984).
W. Verboom, C. Verboom, I. M. Elssink, B. H. M. Lammerink, and D. N. Reinhoudt, Rec. Trav. Chim. Pays-Bas, 109, 481 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Litvinov, V.P., Yakunin, Y.Y. & Dyachenko, V.D. Nucleophilic Vinyl Substitution in the Synthesis of Heterocycles. (Review). Chemistry of Heterocyclic Compounds 37, 37–76 (2001). https://doi.org/10.1023/A:1017536700235
Issue Date:
DOI: https://doi.org/10.1023/A:1017536700235