Abstract
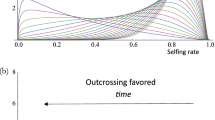
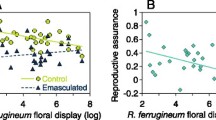
The evolutionarily stable rate of self-fertilzation is studied in phenotypic models that incorporate pollination ecology as well as the correlated evolution of inbreeding depression and the population mean selfing rate. Inbreeding depression is assumed to be caused by continual mutation to deleterious, partially recessive alleles. Several mutation rates and dominance levels are included. Two separate ecological cases are studied: how selfing rate affects proportion of ovules fertilized (pollination assurance, seed discounting) and how selfing rate affects male outcrossing success through pollen discounting. Evolutionarily stable rates are invariably zero or intermediate in two circumstances, namely when increased selfing causes (1) a decrease in the proportion of ovules fertilized or (2) an increase in pollen discounting and, therefore, a disproportionate decrease in male outcrossing success. Complete selfing is stable when selfing increases the proportion of ovules fertilized for all selfing rates. Stable selfing is zero or one in cases where the selfing rate has no effect on the proportion of ovules fertilized or when pollen discounting does not increase with selfing. Higher inbreeding depression tends to decrease the optimal selfing rate, and lower inbreeding depression (higher dominance coefficients and lower mutation rates) is more favorable to the existence of stable intermediate selfing rates. Approaches such as this that explicitly incorporate the interdependence of selfing, ovule fertilization, and male outcrossing may help explain the persistence of intermediate selfing rates in animal-pollinated plants.
Similar content being viewed by others
References
Aide, T.M., 1986. The influence of wind and animal pollination on variation in outcrossing rates. Evolution 40: 434-435.
Barrett, S.C.H. & C.G. Eckert, 1990. Variation and evolution of mating systems in seed plants, pp. 230-254 in Biological Approaches and Evolutionary Trends in Plants. Academic Press Limited.
Campbell, R.B., 1986. The interdependence of mating structure and inbreeding depression. Theor. Popul. Biol. 30: 232-244.
Charlesworth, B., 1980. The cost of sex in relation to mating system. J. Theor. Biol. 84: 655-671.
Charlesworth, B., M.T. Morgan & D. Charlesworth, 1991. Multilocus models of inbreeding depression with synergistic selection and partial self-fertilization. Genet. Res. 57: 177-194.
Charlesworth, D. & B. Charlesworth, 1987. Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 18: 237-268.
Charlesworth, D. & B. Charlesworth, 1990. Inbreeding depression with heterozygote advantage and its effect on selection for modifiers changing the outcrossing rate. Evolution 44: 870-888.
Charlesworth, D., M.T. Morgan & B. Charlesworth, 1990. Inbreeding depression, genetic load, and the evolution of outcrossing rates in amultilocus system with no linkage. Evolution 44: 1469-1489.
Charlesworth, D., M.T. Morgan & B. Charlesworth, 1992. The effect of linkage and population size on inbreeding depression due to mutational load. Genet. Res. 59: 49-61.
Crow, J.F., 1993. Mutation, mean fitness, and genetic load, pp. 342 in Oxford Surveys in Evolutionary Biology, edited by D. J. Futuyma & J. Antonovics. Oxford University Press, New York.
Damgaard, C., D. Couvet & V. Loeschcke, 1992. Partial selfing as an optimal mating strategy. Heredity 69: 289-295.
Endress, P.K., 1994. Diversity and Evolutionary Biology of Tropical Flowers. Cambridge University Press, Cambridge, UK.
Fisher, R.A., 1941. Average excess and average effect of a gene substitution. Ann. Eugen. 11: 53-63.
Fu, Y.B. & K. Ritland, 1994. Evidence for the partial dominance of viability genes contributing to inbreeding depression in Mimulus guttatus. Genetics 136: 323-331.
Harder, L.D. & S.C.H. Barrett, 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512-515.
Holsinger, K.E., 1986. Dispersal and plant mating systems: The evolution of self-fertilization in subdivided populations. Evolution 40: 405-413.
Holsinger, K.E., 1988. Inbreeding depression doesn’t matter: The genetic basis of mating system evolution. Evolution 42: 1235-1244.
Holsinger, K.E., 1991. Mass-action models of plant mating systems: The evolutionary stability of mixed mating systems. Amer. Natur. 138: 606-622.
Holsinger, K.E., 1992 Ecological models of plant mating systems, pp. 169-191 in Ecology and Evolution of Plant Reproductive Systems, edited by R.W. Wyatt. Chapman and Hall, New York.
Holsinger, K.E., 1996. Pollination biology and the evolution of mating systems in flowering plants. Evol. Biol. 29: 107-149.
Holsinger, K.E.,M.W. Feldman & F.B. Christiansen, 1984. The evolution of self-fertilization in plants: A population-genetic model. Amer. Natur. 124: 446-453.
Holtsford, T.P. & N.C. Ellstrand, 1990. Inbreeding effects in Clarkia tembloriensis (Onagraceae) populations with different natural outcrossing rates. Evolution 44: 2031-2046.
Houle, D., D.K. Hoffmaster, S. Assimacopoulos & B. Charlesworth, 1992. The genomic mutation rate for fitness in Drosophila. Nature 359: 58-60.
Husband, B.C.& D.W. Schemske, 1996. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50: 54-70.
Jain, S.K., 1976. The evolution of inbreeding in plants. Annu. Rev. Ecol. Syst. 7: 469-495.
Jarne, P. & D. Charlesworth, 1993. The evolution of selfing rate in functionally hermaphrodite plants and animals. Annu. Rev. Ecol. Syst. 24: 441-466.
Johnston, M.O. & D.J. Schoen, 1995. Mutation rates and dominance levels of genes affecting total fitness in two angiosperm species. Science Wash 267: 226-229.
Johnston, M.O. & D.J. Schoen, 1996. Correlated evolution of self-fertilization and inbreeding depression: An experimental study of nine populations of Amsinckia (Boraginaceae). Evolution 50: 1478-1491.
Keightly, P.D. & A. Caballero, 1997. Genomic mutation rates for lifetime reproductive output and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 94: 3823-3827.
Kondrashov, A.S., 1988. Deleterious mutations and the evolution of sexual reproduction. Nature 336: 435-440.
Lande, R. & S.J. Arnold, 1983. The measurement of selection on correlated characters. Evolution 37: 1210-1226.
Lande, R. & D. W. Schemske, 1985. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution 39: 24-40.
Latta, R. & K. Ritland, 1993. Models for the evolution of selfing under alternative modes of inheritance. Heredity 71: 1-10.
Latta, R. & K. Ritland, 1994a. Conditions favoring stable mixed mating systems with jointly evolving inbreeding depression. J. Theor. Biol. 170: 15-24.
Latta, R. & K. Ritland, 1994b. The relationship between inbreeding depression and prior inbreeding among populations of four Mimulus taxa. Evolution 48: 806-817.
Lloyd, D.G., 1979. Some reproductive factors affecting the selection of self-fertilization in plants. Amer. Natur. 113: 67-79.
Lloyd, D.G., 1983. Evolutionarily stable sex ratios and sex allocations. J. Theoret. Biol. 105: 525-539.
Lloyd, D.G., 1992. Self-and cross-fertilization in plants. II. The selection of self-fertilization. Int. J. Plant Sci. 153: 370-380.
Lloyd, D.G. & D.J. Schoen, 1992. Self-and cross-fertilization in plants. I. functional dimensions. Int. J. Plant Sci. 153: 358-369.
Nagylaki, T., 1976. A model for the evolution of self-fertilization and vegetative reproduction. J. Theoret. Biol. 58: 55-58.
Sakai, S., 1995. Evolutionarily stable selfing rates of hermaphroditic plants in competing and delayed selfing modes with allocation to attractive structures. Evolution 49: 557-564.
Schemske, D.W. & R. Lande, 1985. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution 37: 523-539.
Schemske, D.W. & R. Lande, 1987. On the evolution of plant mating systems. Amer. Natur. 130: 804-809.
Schoen, D.J. & D.G. Lloyd, 1984. The selection of cleistogamy and heteromorphic diaspores. Biol. J. Linn. Soc. 23: 303-322.
Simmons, M.J. & J.F. Crow, 1977. Mutations affecting fitness in Drosophila populations. Ann. Rev. of Genet. 11: 49-78.
Uyenoyama, M.K., K.E. Holsinger & D.M. Waller, 1993. Ecological and genetic factors directing the evolution of self-fertilization. Oxford Surv. Evol. Biol. 9: 327-381.
Uyenoyama, M.K. & D.M. Waller, 1991. Coevolution of self-fertilization and inbreeding depression. II. Symmetric overdominance in viability. Theor. Popul. Biol. 40: 47-77.
Waller, D.M., 1986. Is there disruptive selection for self-fertilization? American Naturalist 128: 421-426.
Williams, G.C., 1975. Sex and Evolution. Princeton Univ. Press, Princeton, N.J.
Wright, S., 1977. Evolution and the Genetics of Populations, Vol. 3. Experimental Results and Evolutionary Deductions. Univ. Chicago Press, Chicago.
Yampolsky, C. & H. Yampolsky, 1922. Distribution of sex forms in the phanerogamic flora. Bibliotheca Genetica 3: 1-62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johnston, M.O. Evolution of intermediate selfing rates in plants: pollination ecology versus deleterious mutations. Genetica 102, 267–278 (1998). https://doi.org/10.1023/A:1017039010191
Issue Date:
DOI: https://doi.org/10.1023/A:1017039010191