Abstract
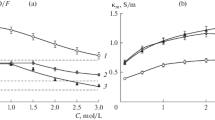
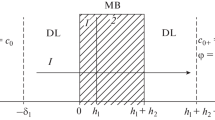
A new method of chronopotentiometric study of electroosmotic water transfer in the electromembrane system containing amino acid solution is elaborated. An electrodiffusion problem, which allows for a convective solvent transfer in the system, is formulated and solved. By comparing calculated and experimental transition times for MK-40 and MF-4SK cationite membranes in lysine monohydrochloride solutions, the electroosmotic permeability of the membranes and transport numbers of water through them are calculated. A considerably higher electroosmotic water transport through MK-40 as compared with MF-4SK is attributed to differences in their nature and structure.
Similar content being viewed by others
REFERENCES
Bobreshova, O.V., Korzhov, E.N., Kharebava, T.Sh., et al., Elektrokhimiya, 1983, vol. 12, p. 1668.
Kharebava, T.Sh., Zubets, N.N., Bobreshova, O.V., et al., Teor. Prakt. Sorbtsionnykh Protsessov, 1983, no. 16, p. 86.
Berezina, N., Gnusin, N., Dyomina, O., and Timofeyef, S., J. Membr. Sci., 1994, vol. 86, p. 207.
Zundel, G., Hydration and Intermolecular Interaction: Infrared Investigations with Polyelectrolyte Membranes, New York: Academic, 1969.
Fioshin, M.M., Shutova, L.A., and Krishtalik, L.I., Elektrokhimiya, 1986, vol. 22, p. 814.
Voitovich, I.M., Shaposhnik, V.A., and Kotov, V.V., Teor. Prakt. Sorbtsionnykh Protsessov, 1976, no. 11, p. 106.
Zabolotskii, V.I., Gnusin, N.P., El'nikova, L.F., and Blednykh, V.M., Zh. Prikl. Khim. (Leningrad), 1986, vol. 59, p. 140.
Bobreshova, O.V., Kulintsov, P.I., Bobrinskaya, G.A., et al., Vestn. Voronezh. Gos. Univ., 2000, no. 6, p. 1.
Zakharov, M.S., Bakanov, V.I., and Pnev, V.R., Khronopotentsiometriya (Chronopotentiometry), Moscow: Khimiya, 1978, p. 116.
Erdey-Grüz, T., Transport Phenomena in Aqueous Solutions, Budapest: Akadémiai Kiadö, 1974.
Damaskin, B.B. and Petrii, O.A., Elektrokhimiya (Electrochemistry), Moscow: Vysshaya Shkola, 1987.
Pevnitskaya, M.V., Kozina, A.A., and Evseev, N.E., Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1974, no. 4, p. 137.
Zabolotskii, V.M. and Nikonenko, V.V., Perenos ionov v membranakh (The Ion Transport in Membranes), Moscow: Nauka, 1996, p. 395.
Gnusin, N.P., Berezina, N.P., Demina, O.A., and Kononenko, N.A., Elektrokhimiya, 1996, vol. 32, p. 173.
Timashev, S.F., Fizikokhimiya membrannykh protsessov (Physical Chemistry of Membrane Processes), Moscow: Khimiya, 1988, p. 236.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Novikova, L.A., Kulintsov, P.I., Bobreshova, O.V. et al. Chronopotentiometric Method of Studying Electroosmosis in Systems with Ion-Exchange Membranes and Lysine Solutions. Russian Journal of Electrochemistry 38, 909–912 (2002). https://doi.org/10.1023/A:1016878115378
Issue Date:
DOI: https://doi.org/10.1023/A:1016878115378