Abstract
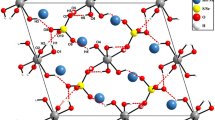
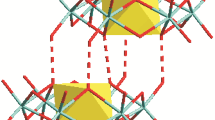
The structure of H3Co2[C5H2(t-Bu)3]2 has been analyzed by low-temperature single-crystal neutron diffraction techniques, and shown to consist of two CoCp‡ moieties with three hydride ligands bridging the central Co–Co bond. Despite a fairly extensive twinning problem, the structure could be solved and successfully refined to a final R factor of 9.2% for 2024 reflections. Average molecular parameters in the H3Co2 core of the molecule are as follows: Co–Co=2.275(21) Å, Co–H=1.637(16) Å, H⋯H=2.050(20) Å, Co–H–Co=88.0(9)°, H–Co–H=77.0(7)°. Also included in this paper is a discussion on the molecular dimensions of symmetric hydride-bridged dinuclear systems (M(μ-H)nM, n=1, 2, 3, 4) that have been studied to date by neutron diffraction.
Similar content being viewed by others
REFERENCES
R. C. Stevens, M. R. McLean, T. Wen, J. D. Carpenter, R. Bau, and T. F. Koetzle (1989). Inorg. Chim. Acta 161, 223.
F. Lutz, R. Bau, P. Wu, T. F. Koetzle, C. Kruger, and J. J. Schneider (1996). Inorg. Chem. 35, 2698.
J. J.Schneider, U. Specht, R. Goddard, and C. Krüger (1997). Chem.Ber. 130, 161.
M. Thomas, R. F. D. Stansfield, M. Berneron, A. Filhol, G. Greenwood, J. Jacobe, D. Feltin, and S. A. Mason, in P. Convert and J. B. Forsyth (eds.), Position-Sensitive Detection of Thermal Neutrons (Academic Press, London, 1983), p. 344.
C. Wilkinson, H. W. Khamis, R. F. D. Stansfield, and G. J. McIntyre (1988). J. Appl. Cryst. 21, 471.
A complete listing of the atomic coordinates, distances, and angles and a summary of the structure analysis can be obtained, on request, by writing to the Cambridge Crystallo-graphic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK (reference number CCDC-148143).
F. Hahn and W. Massa, TWINXL, Programm zur Aufbereitung von Beugungsdaten verzwillingter Kristalle, Version 3.0, (Universität Marburg, Marburg, 1996).
G. M. Sheldrick, SHELX-97, a Computer Program for the Refinement of Crystal Structures (University of Göttingen, Göttingen, 1997).
L. J. Farrugia, WinGX, a Windows Program for Crystal Structure Analysis (University of Glasgow, Glasgow, 1998).
To appreciate why M–M distances in M(µ-H) 3M systems are invariably shorter than M–M distances in M(µ-H) 2M bridges, imagine two octahedra sharing an edge (a situation analogous to M(µ-H)2M) and compare this arrangement with two octahedra sharing a face (analogous to M(µ-H) 3M): If the M–H distances are maintained to be equal in the two cases, the centers of the two octahedra will automatically be closer in the latter case.
A. Dedieu, T. A. Albright, and R. Hoffmann (1979). J. Am. Chem. Soc. 101, 3141.
R. Bau, W. E. Carroll, R. G. Teller, and T. F. Koetzle (1977). J. Am. Chem. Soc. 99, 3872.
J. A. K. Howard, O. Johnson, T. F. Koetzle, and J. L. Spencer (1987). Inorg. Chem. 26, 2930.
L. Brammer, J. A. K. Howard, O. Johnson, T. F. Koetzle, J. L. Spencer, and A. M. Stringer (1991). J. Chem. Soc. Chem. Commun. 241.
S. C. Abrahams, A. P. Ginsberg, T. F. Koetzle, P. Marsh, and C. R. Sprinkle (1986). Inorg. Chem. 25, 2500.
A. Berry, M. L. H. Green, J. A. Bandy, and K. Prout (1991). J. Chem. Soc. Dalton Trans. 2185.
M. H. Drabnis, R. Bau, S. A. Mason, J. W. Freeman, and R. D. Ernst (1998). Eur. J. Inorg. Chem. 851.
J. L. Petersen, R. K. Brown, J. M. Williams, and R. K. McMullan (1979). Inorg. Chem. 18, 3493.
D. W. Hart, R. Bau, and T. F. Koetzle (1985). Organometallics 4, 1590.
C. Y. Wei, M. W. Marks, R. Bau, S. W. Kirtley, D. E. Bisson, M. E. Henderson, and T. F. Koetzle (1982). Inorg. Chem. 21, 2556.
D. Hanke, K. Wieghardt, B. Nuber, R. S. Lu, R. K. McMullan, T. F. Koetzle, and R. Bau (1993). Inorg. Chem. 32, 4300.
R. G. Teller, J. M. Williams, T. F. Koetzle, R. R. Burch, R. M. Gavin, and E. L. Muetterties (1981). Inorg. Chem. 20, 1806.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bortz, M., Bau, R., Schneider, J.J. et al. Neutron Diffraction Analysis of H3Co2[C5H2(t-Bu)3]2, a Molecule with a Triply Hydrogen-Bridged Metal–Metal Bond: Some Comments on Structural Patterns in M(μ-H)nM Systems (n=1, 2, 3, 4). Journal of Cluster Science 12, 285–291 (2001). https://doi.org/10.1023/A:1016643617254
Issue Date:
DOI: https://doi.org/10.1023/A:1016643617254