Abstract
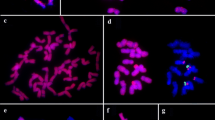
The genomic constitution of the hexaploid Psammopyrum athericum was studied with in-situ DNA hybridization using both genomic DNA and isolated cloned sequences as probes. A genomic probe from Thinopyrum bessarabicum (E genome) hybridized successfully to 14 chromosomes of Ps. athericum and a probe from Festucopsis serpentinii (L genome) hybridized to another 14 chromosomes. The remaining chromosomes did not hybridize, apart from in the centromeric region, to any of the genomic probes used. It is thus proposed that Ps. athericum contains the genomes E, L and X where X stands for a so-far unknown genome. Psammopyrum athericum has three pairs of pTa71 sites and approximately 30 pSc119:2 sites. The origin of the third genome will be a matter for further research using genomic and genome-specific probes.
Similar content being viewed by others
References
Anamthawat-Jonsson K, Reader SM (1995) Pre-annealing of total genomic DNA probes for simultaneous genomic in situ hybridization in cereal species. Genome 38: 81–816.
Anamthawat-Jo¨ nsson K, Heslop-Harrison JS, Schwarzacher T (1996) Genomic in situ hybridization for whole chromosome and genome analysis. In: Clark M, ed. In Situ Hybridization: A Laboratory Companion. London: Chapman & Hall, pp 1-23.
Bothmer von R, Flink J, LandstrÎm T (1986) Meiosis in interspeci¢c Hordeum hybrids I. Diploid combinations. Can J Genet Cytol 28: 52–535.
Bothmer von R, Flink J, LandstrÎm T (1987) Meiosis in Hordeum interspeci¢c hybrids II. Triploids hybrids. Evol Trends Plants 1: 4–50.
Castilho A, Heslop-Harrison JS (1995) Physical mapping of 5S and 18S 25S rDNA and repetitive DNA sequences in Aegilops umbellulata. Genome 38: 9–96.
Cauderon Y (1966) Genome analysis in the genus Agropyron. Hereditas suppl. 2: 21–234.
Dewey DR (1984) The genomic system of classi¢cation as a guide to intergeneric hybridization with the perennial Triticeae. In: Gusta£on JP, ed. Gene Manipulation in Plant Improvement. New York: Plenum Publ. Corp., pp. 20–279.
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7: 186–1885.
Hsiao C, Wang RR-C, Dewey DR (1986) Karyotype analysis and genome relationships of 22 diploid species of the tribe Triticeae. Can J Genet Cytol 28: 10–120.
Humphries CJ (1980) 57. Hordeum L. In: Tutin TGet al., eds Flora Europaea, Cambridge: Cambridge University Press, 5: 20–205.
Jarvie JK, Barkworth ME (1990) Isozyme similarity in Thino-pyrum and its relatives (Triticeae: Gramineae). Genome 33: 88–891.
Jauhar PP (1990)Multidisciplinary approach to genome analy-sis in the diploid species, Thinopyrum bessarabicum and Th. elongatum (Lophopyrum elongatum), of the Triticeae. Theor Appl Genet 80: 52–536.
Kihara H (1930) Genome analysis of Triticum and Aegilops. Cytologia 1: 26–270.
Leendertse PC, Rozema J, Andrea A (1997) Effects of nitrogen addition on the growth of the salt marsh grass Elymus athericus. J Coastal Conservation 3: 3–40.
Linc G, Friebe BR, Kynast RG et al. (1999) Molecular cytogenetic analysis of Aegilops cylindrica Host. Genome 42: 49–503.
Linde-Laursen I, Ibsen E, von Bothmer R, Giese H (1992) Physical localization of active and inactive rRNA gene loci in Hordeum marinum spp. gussoneaeum (4x) by in situ hybridization. Genome 35: 103–1036.
Linde-Laursen I, Seberg O, Frederiksen S, Baden C (1996) The karyotype of Festucopsis serpentinii (Poaceae, Triticeae) from Albania studied by banding techniques and in situ hybridization. Plant Syst Evol 201: 7–82.
Linde-Laursen I, Heslop-Harrison JS, Shepherd KW, Taketa S (1997) The barley genome and its relationship with the wheat genomes. A survey with an internationally agreed recommen-dation for barley chromosome nomenclature. Hereditas 126: 1-16.
Liu Z-W, Wang RR-C (1992) Genome analysis of Elytrigia caespitosa, Lophopyrum nodosum, Pseudoroegneria geni-culata ssp. scytica and Thinopyrum intermedium (Triticeae Gramineae). Genome 36: 10–111.
Liu Z-W, Wang RR-C (1993) Genome constitutions of Thino-pyrum curvifolium, T. scirpeum, T. distichum, and T. junceum (Triticeae: Gramineae). Genome 36(4): 64–651.
LÎve A (1986) Some taxonomical adjustments in eurasiatic wheatgrasses. VerÎff. Geobot. Inst. ETH, Stiftung Rubel, Zurich 87: 4–52.
Lu B-R (1993) Biosystematic investigations of Asiatic wheatgrasses Elymus L. (Triticeae: Poaceae). PhD thesis. The Swedish University of Agricultural Sciences.
Lu B-R, von Bothmer R (1993) Genomic constitution of four Chinese endemic Elymus species: E. brevipes, E. yangii, E. anthosachnoides, and E. altissimus (Triticeae, Poaceae). Genome 36: 86–876.
Majtkowski W (1994) Utilization of grass ecotypes for recultivation. Evaluation and exploitation of genetic resources: prebreeding. Proc Gen Res SectMeeting Eucarpia 1–18 March 1994, Clermond-Ferrand, France. pp 20–215.
McIntyre CL, Clarke BC, Appels R (1988) Ampli¢cation and dispersion of repeated DNA sequences in the Triticeae. Plant Syst Evol 160: 3–59.
McIntyre CL, Pereira S, Moran LB, Appels R (1990) New Sec-ale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33: 63–640.
Melderis A (1980a) 47. Leymus Hochst. In: Tutin TG et al. ed. Flora Europaea, Cambridge: Cambridge University Press, 5: 19–192.
Melderis A (1980b) 48. Elymus L. In: Tutin TG et al. ed. Flora Europaea, Cambridge: Cambridge University Press, 5: 19–198.
Òrgaard M, Anamthawat-Jo¨ nsson K (2000) Genome discrimi-nation in two Elymus and one Elytrigia species (Poaceae: Triticeae) from Iceland by in situ hybridization. Genome (In press).
Òrgaard M, Heslop-Harrison JS (1994) Relationships between species of Leymus Psathyrostachys, and Hordeum (Poaceae, Triticeae) inferred from Southern hybridization of genomic and cloned probes. Plant Syst Evol 189: 21–231.
Schwarzacher T, Leitch A (1994) Enzymatic treatment of plant material to spread chromosomes for in situ hybridization. In: Isaac PG, ed. Methods in Molecular Biology 28: Protocols for Nucleic Acid Analysis by Nonradioactive Probes. Totowa: Humana Press Inc, pp 15–160.
Schwarzacher T, Wang ML, Leitch AR, Miller AR, Moore G, Heslop-Harrison JS (1997) Flow cytometric analysis of the chromosomes and stability of a wheat cell-culture line. Theor Appl Genet 94: 9–97.
Wang RR-C, von Bothmer R, Dvorak J, Fedak G, Linde-Laursen I, Muramatsu M (1997) Genome symbols in the Triticeae (Poaceae). In: Wang RR-C, Jensen KB, Jaussi C, ed. Proceedings of the 2nd International Triticeae Sym-posium, Logan, Utah, pp 2–34.
Rights and permissions
About this article
Cite this article
Ellneskog-Staam, P., Salomon, B., von Bothmer, R. et al. Trigenomic origin of the hexaploid Psammopyrum athericum (Triticeae: Poaceae) revealed by in-situ hybridization. Chromosome Res 9, 243–249 (2001). https://doi.org/10.1023/A:1016604705296
Issue Date:
DOI: https://doi.org/10.1023/A:1016604705296