Abstract
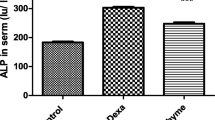
The cytoprotective effects of glycyrrhizin (GL) are similar to glucocorticoids. We investigated the effects of GL on the glucocorticoid receptor (GR) and on the enzyme activity of tyrosine aminotransferase (TAT), an hepatocyte-specific marker of glucocorticoid action, in rat hepatocytes. Pretreatment with GL significantly decreased the affinity of GRs for dexamethasone (DEX) and increased the period of time required for TAT activity to reach a peak after the addition of DEX. GL did not affect the amount of GR, but significantly decreased the amount of heat-shock protein 90 (HSP90) and HSP90-associated GR. Alternatively, TAT activity and TAT mRNA levels increased significantly after the addition of GL to hepatocytes pretreated with DEX. In conclusion, GL reduces the affinity of GRs for ligands through the decreased HSP90 expression, but significantly enhances the glucocorticoid-induced TAT-gene expression at the transcriptional level in rat hepatocytes.
Similar content being viewed by others
REFERENCES
Finney RSH, Somers GF: The anti-inflammatory activity of glycyrrhetinic acid and derivatives. J Pharmacol 10:613–620, 1962
Suzuki H, Ohta Y, Takino T, Fuzisawa K, Hirayama C: Effects of glycyrrhizin on biochemical tests in patients with chronic hepatitis. Asian Med J 26:423–438, 1983
Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H: The long-term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 79:1494–1500, 1997
Rossum TGJ, Vulto AG, Hop WC, Brouwer JT, Niesters HG, Schalm SW: Intravenous glycyrrhizin for the treatment of chronic hepatitis C: A double-blind, randomized, placebocontrolled phase I/II trial. J Gastroenterol Hepatol 14:1093–1099, 1999
Shiki Y, Shirai K, Saito Y, Yoshida S, Mori Y, Wakashin M: Effect of glycyrrhizin on lyses of hepatocyte membranes induced by anti-liver cell membrane antibody. J Gastroenterol Hepatol 7:12–16, 1992
Ohuchi K, Tsurufuji A: A study of the anti-inflammatory mechanism of glycyrrhizin. Minophagen Med Rev 27:188–193, 1982
Yoshikawa M, Matsui Y, Kawamoto H, Umemoto N, Oku K, Koizumi M, Yamao J, Kuriyama S, Nakano H, Hozumi N, Ishizaka S, Fukui H: Effects of glycyrrhizin on immunemediated cytotoxicity. J Gastroenterol Hepatol 12:243–248, 1997
Kumagai A, Yano S, Otomo M: Study on the corticoid-like action of glycyrrhizine and the mechanism of its action. Endocrinol Jpn 4:17–27, 1957
Borst JGG, Holt SP, Vries LA, Molhuysen JA: Synergistic action of liquorice and cortisone in Addison's and Simmonds's disease. Lancet 2:657–663, 1953
Hartl FU: Molecular chaperones in cellular protein folding. Nature 381:571–580, 1996
Aligue R, Akhavan-Niak H, Russell P: A role for HSP90 in cell cycle control: Weel tyrosine kinase activity requires interaction with HSP90. EMBO J 13:6099–6106, 1994
Buchner J: HSP90 & Co.—a holding for folding. Trends Biochem Sci 24:136–141, 1999
Bamberger CM, Schulte HM, Chrousos GP: Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocrinol Rev 17:245–261, 1996
Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR: Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348:166–168, 1990
Tongiani R, Piazzolla S, Paolicchi A: Metyrapone modulation of tyrosine aminotransferase induction by dexamethasone in cultured hepatocytes. Biochem Biophys Res Commun 166:801–806, 1990
Higgins SJ, Rousseau GG, Baxter JD, Tomkins GM: Early events in glucocorticoid action. Activation of the steroid receptor and its subsequent specific nuclear binding studied in a cell-free system. J Biol Chem 248:5866–5872, 1973
Sunahara IG, Lucier GW, Mccoy Z, Bresnick EH, Sanchez ER, Nelson KG: Characterization of 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated decreases in dexamethasone binding to rat hepatic cytosolic glucocorticoid receptor. Mol Pharmacol 36:239–247, 1989
Beato M, Feigelson P: Glucocorticoid-binding proteins of rat liver cytosol. I. Separation and identification of the binding proteins. J Biol Chem 247:7890–7896, 1972
Granner DK, Tomkins GM: Tyrosine aminotransferase (rat liver). Methods Enzymol 17A:633–637, 1970
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254, 1976
Scatchard D: The attraction of protein for small molecules and ions. Ann NY Acad Sci 51:660–672, 1949
Audouin-Chevallier I, Pallet V, Coustaut M, Alfos S, Higueret P, Garcin H: Retinoids modulate the binding capacity of the glucocorticoid receptor and its translocation from cytosol to nucleus in liver cells. J Steroid Biochem Mol Biol 52:321–328, 1995
Kaufman SH, Shaper JH: Binding of dexamethasone to rat liver nuclei in vivo and in vitro: Evidence for two distinct binding sites. J Steroid Biochem 20:699–708, 1984
Cvoro A, Dundjerski J, Trajkovic D, Matic G: Association of the rat liver glucocorticoid receptor with HSP90 and HSP70 upon whole body hyperthermic stress. J Steroid Biochem Mol Biol 67:319–325, 1998
Czar MJ, Owens-Grillo JK, Dittmar KD, Hutchison KA, Zacharek AM, Leach KL, Diebel MR Jr, Pratt WB: Characterization of the protein-protein interactions determining the heat shock protein (hsp90 · hsp70 · hsp56) heterocomplex. J Biol Chem 269:11155–11161, 1994
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685, 1970
Nakayama H, Yokoi H, Fujita J: Quantification of mRNA by non-radioactive RT-PCR and CCD imaging system. Nucleic Acids Res 20:4939, 1992
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159, 1987
Nebes VL, DeFranco D, Morris SMJ: Differential induction of transcription for corticoid-responsive genes in cultured rat hepatocytes. Biochem Biophys Res Commun 166:133–138, 1990
Evans RM: The steroid and thyroid hormone receptor superfamily. Science 240:889–895, 1988
Beato M, Herrlich P, Schütz G: Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857, 1995
Glass CK, Rose DW, Rosenfeld MG: Nuclear receptor coactivators. Curr Opin Cell Biol 9:222–232, 1997
Bresnick EH, Dalman FC, Sanchez ER, Pratt WB: Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J Biol Chem 264:4992–4997, 1989
Morimoto R: Cells in stress: transcriptional activation of heat shock genes. Science 259:1409–1410, 1993
Mitsuyoshi H, Nakashima T, Inaba K, Ishikawa H, Nakajima Y, Sakamoto Y, Matsumoto M, Okanoue T, Kashima K: Ursodeoxycholic acid enhances glucocorticoid-induced tyrosine aminotransferase-gene expression in cultured rat hepatocytes. Biochem Biophys Res Commun 240:732–736, 1997
Dhillon VB, McCallum S, Norton P, Twomey BM, Erkeller-Yuksel F, Lydyard P, Isenberg DA, Latchman DS: Differential heat shock protein overexpression and its clinical relevance in systemic lupus erythematosus. Ann Rheum Dis 52:436–442, 1993
Stahl M, Ludwig D, Fellermann K and Stange EF: Intestinal expression of human heat shock protein 90 in patients with Crohn's disease and ulcerative colitis. Dig Dis Sci 43:1079–1087, 1998
Hudson PB, Mittelman A, Podberezec M: Use of glycyrrhizin after bilateral adrenalectomy. N Engl J Med 251:641–646, 1954
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoh, T., Nakashima, T., Sumida, Y. et al. Effects of Glycyrrhizin on Glucocorticoid Signaling Pathway in Hepatocytes. Dig Dis Sci 47, 1775–1781 (2002). https://doi.org/10.1023/A:1016492527927
Issue Date:
DOI: https://doi.org/10.1023/A:1016492527927