Abstract
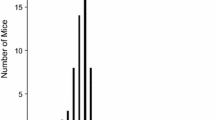
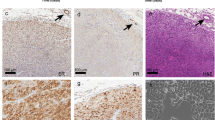
Currently, there is a lack of suitable pre-clinical mouse models for testing and optimization of experimental cancer vaccines. Here, in situ developed mammary tumors of MMTV-v-Ha-ras and MMTV-c-myc transgenic mice and normal mammary, liver, spleen, and testis were screened for expression of tumor-associated antigens (TAA) Mage-b1/2/3 by reverse-transcriptase polymerase chain reaction (RT-PCR) and Southern blot hybridization. Mage-b1/2/3 are homologues of the human TAA MAGE-B1/2/3. Expression of these human MAGE genes has been found in tumors of various histological types, including breast cancer [39]. Mage-specific RT-PCR products (using primers that amplify all three Mage-b1/2/3) were detected in mammary tumors of the MMTV-v-Ha-ras and MMTV-c-myc transgenic mice and in testis, but not in other normal tissues. RT-PCR products obtained from the mammary tumors (using primers that amplify the complete protein-encoding region of Mage-b1/2/3) were cloned and sequenced, and appeared to be most homologous with Mage-b3. Comparison of the Mage-b3 gene in mammary tumors and normal tissues suggest that somatic mutations did not occur in the Mage-b3 gene of the ras- and myc-induced mammary tumors. In addition, no differences were found between the Mage-b3 cDNA of testis, the only normal tissue that expresses Mage-b3, and Mage-b3 in genomic DNA of normal kidney, where Mage-b3 is silent. The MMTV-v-Ha-ras and MMTV-c-myc transgenic mice of this study are the first immune competent mouse models with in situ developed mammary tumors in which the expression of Mage-b3 TAA has been demonstrated. This makes them potentially suitable as a mouse model for pre-clinical testing of Mage-specific cancer vaccines in vivo.
Similar content being viewed by others
References
De Smet C, Lurquin C, Van der Bruggen P, De Plaen E, Brasseur F, Boon T: Sequence and expression pattern of the human MAGE2 gene. Immunogenetics 39: 121–129, 1994
Gaugler B, Van den Eynde B, Van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethe B, Brasseur F, Boon T: Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med 179: 921–930, 1994
Gillespie AM, Rodgers S, Wilson AP, Ti J, Rees RC, Coleman RE, Murray AK: MAGE, BAGE and GAGE: tumour antigen expression in benign and malignant ovarian tissue. Br J Cancer 78: 816–821, 1998
Lethe B, Lucas S, Michaux L, De Smet C, Godelaine D, Serrano A, De Plaen E, Boon T: LAGE-1, a new gene with tumor specificity. Int J Cancer 10: 903–908, 1998
Thompson JA: Molecular cloning and expression of the car-cinoembryonic antigen gene family members. Tumour Biol 16: 10–16, 1995
Disis ML, Cheever MA: Oncogenic proteins as tumor antigensCurr Opin Immunol 8: 637–642, 1996
Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D: Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem 265: 15286–15293, 1990
Coulie PG, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, Boon T: A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci USA 92: 7976–7980, 1995
Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann-Hieb E, De Plaen E, Hankeln T, Meyer zum Buschenfelde KH, Beach D: A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanomaScience 269: 1281–1284, 1995
Robbins PF, El-Gamil M, Li YF, Kawakami Y, Loftus D, Appella E, Rosenberg SA: A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med 183: 1185–1192, 1996
Gravekamp C, Bontenbal M, Ronteltap CP, Van Duyvenbode D, Bolhuis RHL: In vitro and in vivo activation of CD4+ lymphocytes by autologous tumor cells. Int J Cancer 46: 151–152, 1990
Schreiber H: Tumor immunology. In: William E Paul (ed) Fundamental Immunology. 4th edn, Lippincott-Raven Publishers, Philadelphia, 1998, pp 1237–1270
Weiner DB, Kenne RC: Genetic vaccines. Sci Am 281: 50–57, 1999
Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, Lienard D, Beauduin M, Dietrich PY, Russo V, Kerger J, Masucci G, Jager E, De Greve J, Atzpodien J, Brasseur F, Coulie PG, van der Bruggen P, Boon T: Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer 80: 219–230, 1999
Riker A, Cormier J, Panelli M, Kammula U, Wang E, Abati A, Fetsch P, Lee KH, Steinberg S, Rosenberg S, Marincola F: Immune selection after antigen-specific immunotherapy of melanoma. Surgery 126: 112–120, 1999
Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Sznol M, Schwarz SL, Spiess PJ, Wunderlich JR, Seipp CA, Einhorn JH, Rogers-Freezer L, White DE: Impact of cytokine administration on the generation of antitumor reactivity in patients with meta-static melanoma receiving a peptide vaccine. J Immunol 163: 1690–1695, 1999
Chen Q, Jackson H, Cebon J, Gibb P, Davis ID, Trapani JA: A direct comparison of cytolytic T-lymphocyte responses to Melan-A peptides in vitro: differential immunogenicity of Melan-A27–35 and Melan-A26-35. Melanoma Res 10: 16–25, 2000
De Backer O, Verheyden AM, Martin B, Godelaine D, De Plaen E, Brasseur R, Avner P, Boon T: Structure, chromosomal location, and expression pattern of three mouse genes homologous to the human MAGE genes. Genomics 28: 74–83, 1995
De Plaen E, De Backer O, Arnaud D, Bonjean B, Chomez P, Martelange V, Avner P, Baldacci P, Babinet C, Hwang SY, Knowles B, Boon T: A new family of mouse genes homologous to the human MAGE genes. Genomics 55: 176–184, 1999
Kawashima I, Hudson SJ, Tsai V, Southwood S, Takesako K, Appella E, Sette A, Celis E: The multi-epitope approach for immunotherapy for cancer: identification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Human Immunol 59: 1–14, 1998
Fujie T, Mori M, Ueo H, Sugimachi K, Akiyoshi T: Expression of MAGE and BAGE genes in Japanese breast cancers. Ann Oncol 8: 369–372, 1997
Russo V, Traversari C, Verrecchia A, Mottolese M, Natali PG, Bordignon C: Expression of the MAGE gene family in primary and metastatic human breast cancer: implications for tumor antigen-specific immunotherapy. Int J Cancer 64: 216–221, 1995
Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P: Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell 49: 465–475, 1987
Bearss DJ, Subler MA, Hundley JE, Troyer DA, Salinas RA, Windle JJ: Genetic determinants of response to chemotherapy in transgenic mouse mammary and salivary tumorsOncogene 19: 1114–1122, 2000
Gravekamp C, Van de Kemp H, Franzen M, Carrington D, Schoone GJ, Van Eys GJ, Everard CO, Hartskeerl RA, Terpstra WJ: Detection of seven species of pathogenic lepto-spires by PCR using two sets of primers. J Gen Microbiol 139: 1691–1700, 1993
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25: 3389–3402, 1997
Gravekamp C: Tailoring cancer vaccines to the elderly: the importance of suitable mouse models. Mech Age Dev 122: 1087–1105, 2001
Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P: Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54: 105–115, 1988
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF: Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC geneScience 256: 668–670, 1992
Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, Liedtke W, Cohen PE, Kane MF, Lipford JR, Yu N, Crouse GF, Pollard JW, Kunkel T, Lipkin M, Kolodner R, Kucherlapati R: Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell 91: 467–477, 1997
Janitz M, Fiszer D, Jaruzelska J, Simon AK, Biro A, Sachs JA, Kurpisz M: In situ localization of HLA class I mRNA in human testis. Exp Clin Immunogenet 10: 202–207, 1993
Gott JM, Emeson RB: Functions and mechanisms of RNA editing. Annu Rev Genet 34: 499–531, 2000
Loeb LA: A mutator phenotype in cancer. Cancer Res 61: 3230–3239, 2001
Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL: Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 18: 89–95, 1997
Stickeler E, Kittrell F, Medina D, Berget SM: Stage-specific changes in SR splicing factors and alternative splicing in mammary tumorigenesis. Oncogene 18: 3574–3582, 1999
Pharmingen Catalogue 2000, p 1434
Van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boel P, De Smet C, Traversari C, Townsend A, Boon T: A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol 24: 3038–3043, 1994
Lucas S, Brasseur F, Boon T: A new MAGE gene with ubiquitous expression does not code for known MAGE antigens recognized by T cells. Cancer Res 59: 4100–4103, 1999
Lurquin C, De Smet C, Brasseur F, Muscatelli F, Martelange V, De Plaen E, Brasseur R, Monaco AP, Boon T: Two members of the human MAGEB gene family located in Xp21._3 are expressed in tumors of various histological origins. Genomics 46: 397–408, 1997
Van Pel A, De Plaen E, Duffour MT, Warnier G, Uyttenhove C, Perricaudet M, Boon T: Induction of cytolytic T lymphocytes by immunization of mice with an adenovirus containing a mouse homolog of the human MAGE-A genes. Cancer Immunol Immunother 49: 593–602, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sypniewska, R.K., Hoflack, L., Bearss, D.J. et al. Potential Mouse Tumor Model for Pre-Clinical Testing of Mage-Specific Breast Cancer Vaccines. Breast Cancer Res Treat 74, 221–233 (2002). https://doi.org/10.1023/A:1016367104015
Issue Date:
DOI: https://doi.org/10.1023/A:1016367104015