Abstract
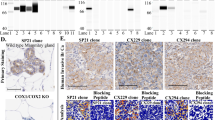
Nitrotyrosine (NO2Y) is a global marker of protein modification by reactive nitrogen species such as peroxynitrite derived from nitric oxide (NO). Because NO and its derivatives are postulated to enhance carcinogenesis, we used stable isotope dilution mass spectrometry to measure the levels of NO2Y in 30 samples of human breast cancer of varying pathologic types. In the samples tested, the NO2Y levels were generally low (average of 14.1 ± 9.2 μmol NO2Y per mole of tyrosine). Breast cancers with a high microvascular density, however, had a significantly higher average level of NO2Y than tumors with a low microvascular density (20 v.s. 10 μmol NO2Y per mole of tyrosine, p = 0.007 by two-tailed t-test, assuming unequal variances of two samples). There was no apparent association between NO2Y levels and the differentiation of the tumors, tumor aneuploidy, estrogen receptor status, HER-2 expression, lymph node status, or infiltration of the tumors by neutrophils or eosinophils. When the tissues were stained by immunohistochemistry for NO2Y, the NO2Y was localized predominantly within inflammatory cells located immediately adjacent to blood vessels at the edges of the tumors. NO2Y was generally not evident within the tumor cells or inflammatory cells in the stroma. We conclude that low levels of reactive nitrogen species are located predominantly within inflammatory cells near blood vessels of breast cancer and that higher NO2Y levels are associated with an increased density of blood vessels. Our findings, therefore, support a possible association between inflammatory cells and reactive nitrogen species in modulating microvascular density at the edges of breast cancer.
Similar content being viewed by others
References
Ekmekcioglu S, Ellerhorst J, Smid C, Prieto V, Munsell M, Buzaid A, Grimm E: Inducible nitric oxide synthase and nitro-tyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res 6: 4768–4775, 2000
Jenkins D, Charles I, Thomsen L, Moss D, Holmes L, Baylis S, Rhodes P, Westmore K, Emson P, Moncada S: Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 92: 4392–4396, 1995
Maeda H, Noguchi Y, Sato K, Akaike T: Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Cancer Res 85: 331–334, 1994
Tozer G, Prise V, Chaplin D: Inhibition of nitric oxide synthase induces a selective reduction in tumor blood flow that is reversible with L-arginine. Cancer Res 57: 948–955, 1997
Thomsen L, Miles D, Happerfield L, Bobrow L, Knowles R, Moncada S: Nitric oxide synthase activity in human breast cancer. Br J Cancer 72: 41–44, 1995
Reveneau S, Arnould L, Jolimoy G, Hilpert S, Lejeune P, SaiNO2 Y-Giorgio V, Belichard C, Jeannin J: Nitric oxide synthase in human breast cancer is associated with tumor grade, proliferation rate, and expression of progesterone receptors. Lab Invest 79: 1215–1225, 1999
Tschugguel W, Schneeberger C, Unfried G, Czerwenka K, Weninger W, Mildner M, Gruber D, Sator M, Waldhor T, Huber J: Expression of inducible nitric oxide synthase in human breast cancer depends on tumor grade. Breast Cancer Res Treat 56: 145–151, 1999
Vakkala M, Kahlos K, Lakari E, Paakko P, Kinnula V, Soini Y: Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin Cancer Res 6: 2408–2416, 2000
Vakkala M, Paakko P, Soini Y: eNOS expression is associated with the estrogen and progesterone receptor status in invasive breast carcinoma. Int J Oncol 17: 667–671, 2000
Beckman J: Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9: 836–844, 1996
Abu-Soud H, Hazen S: Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem 275: 37524–37532, 2000
van der Vliet A, Eiserich J, Halliwell B, Cross C: Formation of reactive nitrogen species during peroxidase-catalyzed ox-idation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem 272: 7617–7625, 1997
Wu W, Chen Y, Hazen S: Eosinophil peroxidase nitrates pro-tein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem 274: 25933–25944, 1999.
Eiserich J, Hristova M, Cross C, Jones A, Freeman B, Halliwell B, van der Vliet A: Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391: 393–397, 1998
Sampson J, Ye Y, Rosen H, Beckman J: Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys 356: 207–213, 1998
van Dalen C, Winterbourn C, Senthilmohan R, Kettle A: Nitrite as a substrate and inhibitor of myeloperoxidase. Implications for nitration and hypochlorous acid production at sites of inflammation. J Biol Chem 275: 11638–11644, 2000
MacPherson J, Comhair S, Erzurum S, Klein D, Lipscomb M, Kavuru M, Samoszuk M, Hazen S: Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol 166: 5763–5772, 2001
Byun J, Henderson J, Mueller D, Heinecke J: 8-nitro-2-deoxyguanosine, a specific marker of oxidation by reactive ni-trogen species, is generated by the myeloperoxidase-hydrogen peroxide-nitrite system of activated human phagocytes. Biochemistry 38: 2590–2600, 1999
Jaiswal M, LaRusso N, Shapiro R, Billiar T, Gores G: Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology 120: 190–199, 2001
Ambs S, Merriam W, Bennett W, Felley-Bosco E, Ogunfusika M, Oser S, Klein S, Shields P, Billiar T, Harris C: Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res 58: 334–341, 1998
Althaus J, Schmidt K, Fountain S, Tseng M, Carroll R, Galatsis P, Hall E: LC-MS/MS detection of peroxynitrite-derived 3-nitrotyrosine in rat microvessels. Free Radic Biol Med 29: 1085–1095, 2000
Loft S, Poulsen HE: Cancer risk and oxidative DNA damage in man. J Mol Med 75: 67–68, 1997
Feig DI, Reid TM, Loeb LA: Reactive oxygen species in tumorigenesis. Cancer Res 54(suppl): 1890s–1894s, 1994
Cerutt PA: Prooxidant states and tumor promotion. Science 227: 375–381, 1985
Shen Z, Mitra SN, Wu W, Chen Y, Yang Y, Qin J, Hazen SL: Eosinophil peroxidase catalyzes bromination of free nucleos-ides and double-stranded DNA. Biochemistry 40: 2041–2051, 2001
Henderson JP, Byun J, Mueller DM, Heiniecke JM: The eosinophil peroxidase hydrogen peroxide-bromide system of human eosinophils generates 5-bromouracil, a mutagenic thymine analogue. Biochemistry 40: 2052–2059, 2001
Henderson JP, Byun J, Williams MV, McCormick ML, Parks WC, Ridnour LA, Heinecke JW: Bromination of deoxycytodine by eosinophil peroxidase: A mechanism for mutagenesis by oxidative damage of nucleoside precursors. Proc Natl Acad Sci USA 98: 1631–1636, 2001
Shen Z, Wu W, Hazen SL: Activated leukocytes oxidatively damage DNA, RNA, and the nucleotide pool through halide-dependent formation of hydroxyl radical. Biochemistry 39: 5474–5482, 2000
Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ: Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 60: 184–190, 2000
Robinson EK, Sneige N, Grimm EA: Correlation of interleukin 6 with interleukin 1 alpha in human mammary tumours, but not with oestrogen receptor expression. Cytokine 10: 970–976, 1998
Sotirious C, Lacroix M, Lespagnard L, Larsimont D, Paesmans M, Body JJ: Interleukins-6 and-11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett 169: 870–895, 2001
Fontanini G, Campani D, Roncellar M, Cecchetti D, Calvo S, Toniolo A, Basolo F: Expression of interleukin 6 (IL-6) correlates with oestogen receptor in human breast carcinoma. Br J Cancer 80: 579–584, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Samoszuk, M., Brennan, ML., To, V. et al. Association Between Nitrotyrosine Levels and Microvascular Density in Human Breast Cancer. Breast Cancer Res Treat 74, 271–278 (2002). https://doi.org/10.1023/A:1016328526866
Issue Date:
DOI: https://doi.org/10.1023/A:1016328526866