Abstract
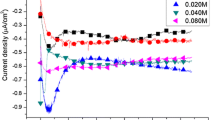
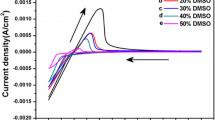
CdS thin films of about 1 μm thickness were deposited from an aqueous solution containing Cd2+, Na2S2O3 and gelatin as the protective colloid to stabilize the size of colloidal sulfur at from 30 to 40 nm and keep the concentration to an appropriate value during electrolysis. The effects of concentrations of Cd2+ and S2O3 2− ions and the deposition potential on the composition of CdS films were studied. The reaction mechanism of CdS film formation on the electrode is discussed. CdS film, whose composition is uniform across the film and which does not contain excess metallic cadmium, can be deposited from a solution containing 0.50 to 2.00 mM Cd(NO3)2, 1.00 to 5.00 mM Na2S2O3 and 1.0 × 10−7 to 1.0 × 10−3 wt % gelatin.
Similar content being viewed by others
References
S. Wagner, J.L. Shay, K.J. Bachmann and E. Buehler, Appl. Phys. Lett. 26 (1975) 229.
K. Mitchell, A.L. Fahrenbruch and R.H. Bube, J. Appl. Phys. 48 (1977) etc. 4365.
K. Yamaguchi, N. Nakayama, H. Matsumoto and S. Ikegami, Jpn. J. Appl. Phys. 16 (1977) 1203.
L.C. Isett, J. Appl. Phys. 55 (1984) 3190.
N.R. Pavaskar, C.A. Menezes and A.P.B. Sinha, J. Electrochem. Soc. 124 (1977) 743.
H. Hirata and K. Higashiyama, Fresenius’ Z. Anal. Chem. 257 (1971) 104.
M. Bettini, K.J. Bachmann and E. Buehler, J. Appl. Phys. 48 (1977) 1603.
J. Humenberger, G. Linnert and K. Lischka, Thin Solid Films 121 (1984) 75.
G.P. Power, D.R. Peggs and A.J. Parker, Electrochim. Acta 26 (1981) 681.
J.F. McCann and M.S. Kazacos, J. Electroanal. Chem. 119 (1981) 409.
B.M. Basol, Solar Cells 23 (1988) 69.
S.K. Das and G.C. Morris, Solar Energy Materials and Solar Cells 28 (1993) 305.
B.E. McCandal, A. Mondal and R.W. Birkmire, Solar Energy Materials and Solar Cells 36 (1995) 369.
E. Fatas, P. Herrasti, F. Arjona, E.G. Camarero and J.A. Medina, Electrochim. Acta 32 (1987) 139.
E. Fatas and P. Herrasti, Electrochim. Acta 33 (1988) 959.
S. Preusser and M. Cocivera, J. Electroanal. Chem. 252 (1988) 139.
R. Flood, B. Enright, M. Allen, S. Barry, A. Dalton, H. Doyle, D. Tynan and D. Fitzmaurice, Solar Energy Materials and Solar Cells 39 (1995) 83.
E. Fatas, P. Herrasti, F. Arjona and E.G. Camarero, J. Electrochem. Soc. 134 (1987) 2799.
E.M. Zaiserand V.K. La Mer, J. Colloid Sci. 3 (1948) 571.
ASTM X-Ray PowderData 5–674.
ASTM X-Ray PowderData 22–829.
K. Siegbahn, C. Nordling, A. Fahlman, R. Nordberg, K. Harmrin, J. Hadman, G. Johansson, T. Bergmark, S.E. Karlsson, I. Lindgren and B. Lindberg, ‘ESCA, Atomic, Molecular and Solid State Structure Studied by Means of Electron Spectroscopy’, Royal Society of Science of Uppsala (1965).
A.S. Baranski and W.R. Fawcett, J. Electrochem. Soc. 131 (1984).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahashi, M., Hasegawa, S., Watanabe, M. et al. Preparation of CdS thin films by electrodeposition: effect of colloidal sulfur particle stability on film composition. Journal of Applied Electrochemistry 32, 359–367 (2002). https://doi.org/10.1023/A:1016325211708
Issue Date:
DOI: https://doi.org/10.1023/A:1016325211708