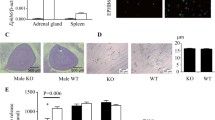
Abstract
The adrenal gland of the lizard Podarcis sicula is formed by a dorsal ribbon of chromaffin cells, generally defined as medullary tissue, arranged along a central part of steroidogenic cells considered as cortical tissue. These two tissues produce catecholamines and steroids as part of the hypothalamo-hypophyseal-adrenal gland axis. Recent studies have demonstrated that Podarcis sicula adrenal gland is not only under hypothalamo-hypophyseal axis control but that several peptides may influence the physiological activity of the gland; among these, vasoactive intestinal peptide is able to enhance strongly both catecholamine and steroid hormone production. The aim of the present study was to verify whether vasoactive intestinal peptide administration could become deleterious. For this reason, we monitored the pattern of expression of two members of the Bcl-2 family, Bcl-2 and Bax, in control and vasoactive intestinal peptide treated specimens. Furthermore, we also tested if peptide treatment induces apoptosis by TUNEL assay.
Similar content being viewed by others
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326.
Alnemri ES, Robertson NM, Fernandes TF, Croce CM, Litwack G (1992) Overexpressed full-length human Bcl2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci USA 89: 7295–7299.
Baldi A, Calia E, Ciampini A, Riccio M, Vetuschi A, Persico AM, Keller F (2000) Deafferentation-induced apoptosis of neurons in thalamic somatosensory nuclei of the newborn rat: critical period and rescue from cell death by peripherally applied neurotrophins. Eur J Neurosci 12: 2281–2290.
Chen-Levy Z, Nourse J, Cleary M(1989) The bcl-2 candidate protooncogene product is a 24–kilodalton integral membrane protein highly expressed in lymphoid cell lines and lymphoma carrying the t(14;18) translocation. Mol Cell Biol 9: 701–710.
Chinnaiyan AM, O'rourke K, Lane BR, Dixit VM (1997) Interaction of CED-4 with CED-3 and CED-9: A molecular framework for cell death. Science 275: 1122–1126.
Craig RW (1995) The bcl-2 gene family. Semin Cancer Biol 6: 35–43.
Davies JC (1995) The bcl-2 family of proteins, and the regulation of cell survival. Trends Neurol Sci 18: 355–358.
De Falco M, Valiante S, Sciarrillo R, Varano L, Laforgia V (2001a) Endothelin 1 (ET-1) control on the adrenal gland activity of the lizard Podarcis sicula. Perspective in Comparative Endocrinology, 14th International Congress of Comparative Endocrinology, 887–892.
De Falco M, Sciarrillo R, Capaldo A, Laforgia V, Varano L, De Luca A (2001b) Shift from noradrenaline to adrenaline production, in lizard adrenal gland, after vasoactive intestinal peptide (VIP) stimulation (submitted).
De Luca A, Russo P, Severino A, Baldi A, Battista T, Cavallotti I, De Luca L, Baldi F, Giordano A, Paggi MG (2001a) Pattern of expression of cyclin T1 in human tissues. J Histochem Cytochem 49: 685–692.
De Luca A, Tosolini A, Russo P, Severino A, Baldi A, De Luca L, Cavallotti I, Baldi F, Giordano A, Testa JR, Paggi MG (2001b) Cyclin T2a gene maps on human chromosome. J Histochem Cytochem 49: 693–698.
Gillardon F, Wickert H, Zimmerman M(1995) Up-regulation of Bax and down regulation of Bcl-2 is associated with kainite-induced apoptosis in mouse brain. Neurosci Lett 192: 85–88.
Gillardon F, Lenz C, Waschke KF, Krajewski S, Reed JC, Zimmerman M, Kuschinsky W (1996) Altered expression of Bcl-2, Bcl-X, Bax, and c-Fos colocalizes with DNA fragmentation and ischemic cell damage following middle cerebral artery occlusion in rats. Mol Brain Res 40: 254–260.
Goldstein P (1997) Controlling cell death. Science 275: 1081–1082.
Hockenberry DM, Zutter M, Hickey W, Nahm M, Korsmeyer S (1991) Bcl-2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 88: 6961–6965.
Hsu YT, Youle RJ (1997) Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272: 13829–13834.
Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC(1993) Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 361: 365–368.
Krajewski S, Mal JK, Krajewski M, Sikorska M, Mosakowski MJ, Reed JC (1995) Upregulation of Bax protein levels in neurons following cerebral ischemia. J Neurosci 15: 6364–6376.
Laforgia V, Varano L (1978) The influence of the interrenal steroidogenic tissue on the chromaffin cells of the adrenal gland of Lacerta s. sicula Raf.: Effects of ACTH administration during winter. Cell Mol Biol 23: 379–390.
Laforgia V, Muoio R (1997) Effects of ACTH and corticosteroids on Phenylethanolamine-N-methyltransferase (PNMT) expression as determined by immunocytochemical localization in the adrenal gland of the lizard Podarcis sicula. Ital J Zool 64: 301–306.
Leboulenger F, Leroux P, Delarue C, Tonon MC, Charnay Y, Dubois PM, Coy DH, Vaudry H (1983) Co-localization of vasoactive intestinal peptide (VIP) and enkephalin in chromaffin cells of the adrenal gland of amphibia. Stimulation of corticosteroid production by VIP. Life Sci 32: 375–383.
Lutz RJ (2000) Role of the BH3 (Bcl-2 homology 3) domain in the regulation of apoptosis and Bcl-2–related proteins. Biochem Soc Trans 28: 51–56.
Malhotra RK, Wakade AR(1987)Vasoactive intestinal polypeptide stimulates the secretion of catecholamines from the rat adrenal gland. J Physiol 388: 285–294. Manzo C, Zerani M, Gobetti A, Di Fiore MM, Angelini F (1994) Is corticosterone involved in the reproductive processes of the male lizard, Podarcis s. sicula?. Hormones Behav 28: 117–129. Mazzocchi G, Malendowicz LK, Nussdorfer GG (1994) Stimulatory effect of vasoactive intestinal peptide (VIP) on the secretory activity of dispersed rat adrenocortical cells. Evidence for the interaction of VIP with ACTH receptors. J Steroid Biochem Molec Biol 48: 507–510. Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ (1994) Bcl2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development 120: 301–311. Oliv´e M, Ferrer I (2000) Bcl-2 and Bax immunohistochemistry in denervation-reinnervation and necrosis-regeneration of rat skeletal muscles. Muscle Nerve 23: 1862–1867. Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell 74: 609–619. Ravishankar S, Ashraf QM, Fritz K, Mishra OP, Delivoria-Papadopoulos M (2001) Expression of Bax and Bcl-2 proteins during hypoxia in cerebral cortical neuronal nuclei of newborn piglets: Effects of administration of magnesium sulfate. Brain Res 901: 23–29. Reed JC (1997) Double identity for proteins of the Bcl-2 family. Nature 387: 773–776. Reed JC (1998) Bcl-2 family proteins. Oncogene 17: 3225–3236. Said SI, Mutt V (1970) Polypeptide with broad biological activity: Isolation from small intestine. Science 169: 1217. Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA 92: 7834–7838. Silke J, Vaux DL (1998) Cell death: Shadow Baxing. Curr Biol 8: R528–R531. Varano L, Laforgia V, Putti R, Cavagnuolo A (1988) Insulin induced modifications in the adrenal chromaffin cells of the lizard Podarcis s. sicula Raf. Arch. It. Anat Embriol 93: 15–28. Varano L, Laforgia V (1991) Evolutionary trends in the adrenal gland of reptiles. In: Ghiara G et al., eds. Symposium on the Evolution of Terrestrial Vertebrates. Selected Symposia and Monographs U.Z.I., 4, Mucchi, Modena, pp. 291–303. Virgolini I, Kurtaran A, Raderer M, Leimer M, Angelberger P, Havlik E, Li S, Scheithauer W, Niederle B, Valent P, et al. (1995) Vasoactive intestinal peptide receptor scintigraphy. J Nucl Med 36: 1732–1739. Yamamoto CM, Sinha Hikim AP, Huynh PN, Shapiro B, Lue Y, Salameh WA, Wang C, Swerdloff RS (2000) Redistribution of Bax is an early step in an apoptotic pathway leading to germ cell death in rats, triggered by mild testicular hyperthermia. Biol Reprod 63: 1683–1690.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Falco, M., Laforgia, V., Fedele, V. et al. Vasoactive Intestinal Peptide Stimulation Modulates the Expression of Bcl-2 Family Members in the Adrenal Gland of the Lizard Podarcis Sicula . Histochem J 33, 639–645 (2001). https://doi.org/10.1023/A:1016302400996
Issue Date:
DOI: https://doi.org/10.1023/A:1016302400996