Abstract
Purpose. To use the drug kinetics in dermis to predict the in vivo blood concentration after topical administration.
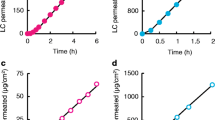
Methods. A two-step pharmacokinetic model was established. The first step was to calculate the drug input rate or flux from the skin to the systemic circulation using the drug kinetic parameters in dermis. These parameters include (a) distance over which the drug concentration declines by 50%, (b) drug concentration at the epidermal-dermal junction, and (c) minimal plateauing drug concentration in the muscle layer. These parameters were experimentally determined from the drug concentration-tissue depth profiles in the dermis, after the application of a topical dose of ddI (200 mg/kg) to rats. The second step was to use the drug input rate together with the systemic disposition pharmacokinetics of ddI in rats to predict the plasma concentration-time profiles. The model-predicted plasma concentration-time profiles were compared with the observed profiles, to determine the validity of the proposed pharmacokinetic model.
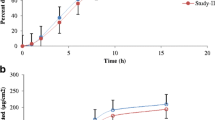
Results. The observed steady state concentration (Css) in individual animals (n = 6) deviated from the predicted values by 3 to 55% with 3 of 6 rats showing a <15% deviation. The mean observed Css of all animals deviated from the mean predicted values by less than 15%.
Conclusions. The close agreement between the observed and the model-predicted drug concentrations indicates that the systemic drug input can be calculated from the drug kinetics in the dermis.
Similar content being viewed by others
REFERENCES
A. C. Williams and B. W. Barry. Skin absorption enhancer. Critical Rev. Therap. Drug Carrier Systems 9:305–353 (1992).
R. H. Guy and J. Hadgraft. Mathematical models of percutaneous absorption. In R. L. Bronaugh and H. I. Maibach (eds.), Percutaneous absorption, Marcel Dekker, Inc. New York, 1989, pp. 13–26.
N. L. Benowitz, P. Jacob, P. Olsson, and C.-J. Johansson. Intravenous nicotine retards transdermal absorption of nicotine: Evidence of blood flow-limited percutaneous absorption. Clin. Pharmacol. Ther. 52:223–230, 1992.
L. K. Pershing, S. Huether, R. L. Conklin, and G. G. Krueger. Cutaneous blood flow and percutaneous absorption: a quantitative analysis using a laser doppler velocimeter and a blood flow meter. J. Invest. Dermatol. 92:355–359 (1989).
J. E. Riviere and P. L. Williams. Pharmacokinetic implication of changing blood flow in skin. J. Pharm. Sci. 81:601–602 (1992).
P. Singh and M. S. Roberts. Blood flow measurements in skin and underlying tissues by microsphere method: application to dermal pharmacokinetics of polar nonelectrolytes. J. Pharm. Sci. 82:873–879 (1993).
M. F. Flessner, R. L. Dedrick, and J. S. Schultz. A distributed model of peritoneal-plasma transport analysis of experimental data in the rat. Am. J. Physiol. 241:F413–F424 (1985).
M. G. Wientjes, J. T. Dalton, R. A. Badalment, J. R. Drago, and J. L.-S. Au. Bladder wall penetration of intravesical mitomycin C in dogs. Cancer Res. 51:4347–4354 (1991).
E. Gupta, M. G. Wientjes, and J. L.-S. Au. Penetration kinetics of 2′,3′-dideoxyinosine in dermis is described by the distributed model. Pharm. Res. 11:809–815 (1994).
B. P. Imbimbo, P. Martinelli, M. Rocchettit, G. Ferrari, G. Bassotti, and E. Imbimbo. Efficiency of different criteria for selecting pharmacokinetic multiexponential equations. Biopharm. Drug Disp. 12:139–147 (1991).
J. S. Schultz and W. Armstrong. Permeability of interstitial space of muscle (rat diaphragm) to solutes of different molecular weights. J. Pharm. Sci. 67:696–705 (1978).
E. M. Renkin. Multiple pathways of capillary permeability. Circ. Res. 41:735–751 (1977).
E. Mukherji. Delivery and pharmacodynamics of dideoxynucleosides. Doctoral Dissertation. Ohio State University, Columbus, Ohio (1993).
M. Gibaldi and D. Perrier. Pharmacokinetics, Marcel Dekker, Inc., New York, 1975.
H. Schaefer and A. Zesch. Penetration of vitamin A into human skin. Acta. Derm. Venereol. 74(c):50–55 (1975).
Rights and permissions
About this article
Cite this article
Gao, X., Wientjes, M.G. & Au, J.LS. Use of Drug Kinetics in Dermis to Predict in VivoBlood Concentration After Topical Application. Pharm Res 12, 2012–2017 (1995). https://doi.org/10.1023/A:1016268628647
Issue Date:
DOI: https://doi.org/10.1023/A:1016268628647