Abstract
Purpose. The three-points method was newly developed by sampling the portal and hepatic veins and systemic artery. A model of hepatic local disposition with the Michaelis-Menten elimination was proposed to explain the concentration dependency of the hepatic recovery ratio (F H).
Methods. 5-fluorouracil (5-FU) was selected as a model drug. 5-FU was administered orally 90 min after its intraarterial dose. Blood specimens in both femoral artery and hepatic vein were sampled after intraarterial dose, and blood specimens in both femoral artery and portal vein were taken after oral administration.
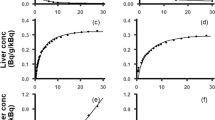
Results. It was shown that F H increased with an increase in the input drug concentration into the liver. The mean absorption time (MAT) estimated by nonlinear analysis agreed with the mean local absorption time (t a) whereas MAT by linear analysis was significantly smaller than t a.
Conclusions. The three-points method was newly developed, and the proposed nonlinear model explained well the capacity-limited elimination of 5-FU through the liver. MAT by the nonlinear analysis was in good agreement with t a.
Similar content being viewed by others
REFERENCES
H. Yasui, K. Yamaoka, T. Fukuyama, and T. Nakagawa. Effect of liver intoxication by carbon tetrachloride on hepatic local disposition of oxacillin using moment characteristics as index. Drug Metab. Dispos. 23:779–785 (1995).
W. Tang, R. A. Stearns, G. Y. Kwei, S. A. Iliff, R.R. Miller, M.A. Egan, N.X. Yu, D.C. Dean, S. Kumar, M. Shou, J.H. Lin, and T. A. Baillie. Interaction of diclofenac and quinidine in monkeys: Simulation of diclofenac metabolism. J. Pharmacol. Exp. Ther. 291:1068–1074 (1999).
T. Mushiroda, R. Douya, E. Takahara, and O. Nagata. The involvement of flavin-containing monooxygenase but not CYP3A4 in metabolism of itopride hydrochloride, a gastroprokinetic agent: Comparison with cisapride and mosapride citrate. Drug Metab. Dispos. 28:1231–1237 (2000).
E. Arlander, G. Ekstrom, C. Alm, J. A. Carrillo, M. Bielenstein, Y. Bottiger, L. Bertilsson, and L. L. Gustafsson. Metabolism of ropivacaine in humans is mediated by CYP1A2 and to a minor extent by CYP3A4: An interaction study with fluvoxamine and ketoconazole as in vivo inhibitors. Clin. Pharmacol. Ther. 64:484–491 (1998).
H. Nakayama, T. Kinouchi, K. Kataoka, S. Akimoto, Y. Matsuda, and Y. Ohnishi. Intestinal anaerobic bacteria hydrolyse sorivudine, producing the high blood concentration of 5-(E)-(2-bromovinyl)uracil that increases the level and toxicity of 5-fluorouracil. Pharmacogenetics 7:35–43 (1997).
T. Watabe, H. Okuda, and K. Ogura. Lethal drug interactions of the new antiviral sorivudine, with anticancer prodrugs of 5-fluorouracil. Yakugaku Zasshi 117:910–921 (1997).
H. Okuda, T. Nishiyama, Y Ogura, S. Nagayama, K. Ikeda, S. Yamaguchi, Y. Nakamura, K. Kawaguchi, and T. Watabe. Lethal drug interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. Drug Metab. Dispos. 25:270–273 (1997).
T. Yamada, K. Niinuma, M. Lemaire, T. Terasaki, and Y. Sugiyama. Carrier-mediated hepatic uptake of the cationic cyclopeptide, octretide, in rats comparison between in vivo and in vitro. Drug Metab. Dispos. 25:536–543 (1997).
R. M. Bremnes, L. Slordal, E. Wist, and J. Aarbakke. Dosedependent pharmacokinetics of methotrexate and 7-hydroxymethotrexate in the rat in vivo. Cancer Res. 49:6359–6364 (1989).
R. F. Frye, A. Adedoyin, K. Mauro, G. R. Matzke, and R. A. Branch Use of chlorzoxazone as an in vivo probe of cytochrome P450 2E1: Choice of dose and phenotypic trait measure. J. Clin. Pharmacol. 38:82–89 (1998).
K. Tabata, K. Yamaoka, T. Fukuyama, and T. Nakagawa. Evaluation of intestinal absorption into the portal system in enterohepatic circulation by measuring the difference in portal-venous blood concentrations of diclofenac. Pharm. Res. 12:880–883 (1995).
D. J. Hoffman, T. Seifert, A. Borre, and H. N. Nellans. Method to estimate the rate and extent of intestinal absorption in conscious rats using an absorption probe and portal blood sampling. Pharm. Res. 12:889–894 (1995).
Y. Fujieda, K. Yamaoka, T. Ito, and T. Nakagawa. Local absorption kinetics of levofloxacin from intestinal tract into portal vein in conscious rat using portal-venous concentration difference. Pharm. Res. 13:1201–1204 (1996).
Y. Sawai, K. Yamaoka, A. Takemura, and T. Nakagawa. Moment analysis of intestinal first-pass metabolism by portal-systemic concentration difference in single conscious rat using 5'-deoxy-5-fluorouracil as model drug system. J. Pharm. Sci. 86:1269–1272 (1997).
T. Ito, K. Yamaoka, and T. Nakagawa. Short-period doubledosing for simultaneous evaluation of intestinal absorption and hepatic disposition in a single conscious rat using cephalexin as test drug. J. Pharm. Pharmacol. 49:1189–1194 (1997).
S. Ueda, K. Yamaoka, T. Nakagawa. Effect of pentobarbital anesthesia on intestinal absorption and hepatic first-pass metabolism of oxacillin in rats, evaluated by portal-systemic concentration difference. J. Pharm. Pharmacol. 51:585–589 (1998).
Y. Sawai, K. Yamaoka, T Ito, and T. Nakagawa. Simultaneous evaluation of intestinal absorption and hepatic extraction of 5-fluorouracil using portal-systemic concentration difference by short-period double dosing in a single conscious rat. Biol. Pharm. Bull. 20:1313–1316 (1997).
M. Higashimori, K. Yamaoka, and T. Nakagawa. Dose-dependency in local disposition of 5-fluorouracil under non-steadystate condition in rat liver. J. Pharm. Sci. 89:100–107 (2000).
B. E. Harris, R. L. Song, S. J. Soong, and R.B. Diasio. Circadian variation of 5-fluorouracil catabolism in isolated perfused rat liver. Cancer Res. 49:6610–6614 (1989).
J. Nishigaki, S. Suzuki, J. Yui, and A. Shigematsu. Distribution volume of three 99mTc-labeled compounds in the rat liver with time after intraportal and intravenous injections. Biol. Pharm. Bull. 18:1705–1709 (1995).
J. Nishigaki, Y. Suzuki, and A. Shigematsu. A novel method for measuring the hepatic first-pass effect and metabolic rate of L-3,4-dihydroxyphenylalanine (DOPA), diazepam and inulin in rat liver. Biol. Pharm. Bull. 21:735–740 (1998).
K. Yamaoka, T. Nakagawa, and T. Uno. Statistical moments in pharmacokinetics. J. Pharmacokinet. Biopharm. 6:547–558 (1978).
K. B. Bischoff, R. L. Dedrick, D. S. Zaharko, and J. A. Longstreth. Methotrexate pharmacokinetics. J. Pharm. Sci. 60:1128–1133 (1971).
K. Yamaoka, Y. Tanigawara, T. Nakagawa, and T. Uno. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharmaco. Dyn. 4:879–885 (1981).
M. Weiss and W. Forster. Pharmacokinetic model based on circulatory transport. Eur. J. Clin. Pharmacol. 16:287–293 (1979).
M. Weiss. Definition of pharmacokinetic parameters: Influence of the sampling site. J. Pharmacokinet. Biopharm. 11:63–75 (1983).
K.C. Kwan. Oral bioavailability and first-pass effects. Drug Metab. Dispos. 25:1329–1336 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, S., Yamaoka, K., Yui, J. et al. Evaluation of Capacity-Limited First-Pass Effect through Liver by Three-Points Sampling in Portal and Hepatic Veins and Systemic Artery. Pharm Res 19, 852–857 (2002). https://doi.org/10.1023/A:1016165101921
Issue Date:
DOI: https://doi.org/10.1023/A:1016165101921