Abstract
Purpose. To quantify the extent to which a sex-specific dichotomy in the temporal evolution of the analgesic effect, after intravenous (i.v.) methadone injection in the rat, relates to the pharmacokinetics (PK) and pharmacodynamics (PD) that mediate the dose-to-effect pathway.
Methods. Tail-flick analgesia was measured after i.v. methadone injection (0.35 mg/kg) in female (n = 16) and male (n = 16) Sprague-Dawley rats. The PK were evaluated in separate female (n = 56) and male (n = 56) rats after they had received the same dose of methadone i.v. (0.35 mg/kg). A bicompartmental model described the kinetics and a sigmoid Emax model-related drug effect vs. simulated concentrations (pharmacodynamics) at the times of effect measurement. All model parameters as well as interanimal and assay variabilities were estimated with a mixed-effects population method using the program NONMEM.
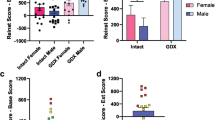
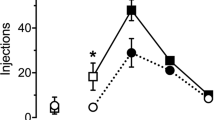
Results. The area under the effect-time curve (AUCE0-120) was (mean ± interanimal SD) 1859 ± 346 min in the females, which was significantly lower than the 4871 ± 393 min in the males (P < 0.0001). On the contrary, the profiles of concentration vs. time were higher in females and, therefore, corresponded inversely to the effect vs. time-relative magnitudes. The central volume of distribution, V1, was 1.94 ± 0.37 l/kg for female rats and 3.01 ± 0.33 l/kg for male rats. Also, the central clearance was 0.077 ± 0.006 l/min/kg and 0.102 ± 0.005 l/min/kg, respectively, for female and male rats. Both parameters differed significantly between sexes (P < 0.0001). The pharmacodynamic maximum observed effect parameter (Emax) was 37% ± 29% in female rats and 85% ± 16% in male rats, and these values were significantly different (P < 0.0001). The parameter for the concentration eliciting half of Emax (EC50) was 24.1 ± 7.5 μg/l in female rats and 20.3 ± 2.9 μg/l in male rats, and the Hill-related exponent, γ, was 6.3 ± 3.9 in female rats and 5.5 ± 4.1 in male rats. These parameters did not differ significantly (at the P < 0.05 level).
Conclusions. A sex-specific dichotomy in the methadone antinociceptive effect, in the rat, was not proportionally related to plasma concentrations. Each sex corresponded to a distinct subpopulation of the PK parameters and one of the pharmacodynamic parameters (Emax). When the course of a drug involves PK or PD subpopulations, PK/PD modeling can afford the safest prediction of the effect-time evolution for a particular dose.
Similar content being viewed by others
REFERENCES
A. I. Baamonde, A. Hidelgo, and F. Andres-Trelles. Sex related differences in the effects of morphine and stress on visceral pain. Neuropharmacology 28:967–970 (1989).
T. J. Cicero, B. Nock, and E. R. Meyer. Gender-related differences in the antinociceptive properties of morphine. J. Pharmacol. Exp. Ther. 279:767–773 (1996).
T. J. Cicero, B. Nock, and E. R. Meyer. Sex-related differences in morphine´ s antinociceptive activity: Relationship to serum and brain morphine concentration. J. Pharmacol. Exp. Ther. 282:939–944 (1997).
B. Kest, E. Sarton, and A. Dahan. Gender differences in opioidmediated analgesia. Anesthesiology 93:539–547 (2000).
R. Z. Harris, L. Z. Benet, and J. B. Schwartz. Gender effects in pharmacokinetics and pharmacodynamics. Drugs 50:222–239 (1995).
P. A. Thurmann and B. C. Hompesch. Influence of gender on the pharmacokinetics and pharmacodynamics of drugs. Int. J. Clin. Pharmacol. Ther. 36:586–590 (1998).
B. Beierle. Meibohm, and H. Derendorf. Gender differences in pharmacokinetics and pharmacodynamics. Int. J. Clin. Pharmacol. Ther. 37:529–547 (1999).
P. L. Bonate. Gender-related differences in xenobiotic metabolism. J. Clin. Pharmacol. 31:684–690 (1991).
R. Kato and Y. Yamazoe. Sex-specific cytochrome P-450 as a cause of sex-and species-related differences in drug toxicity. Toxicol. Lett. 65:661–667 (1992).
E. Tanaka. Gender-related differences in pharmacokinetics and their clinical significance. J. Clin. Pharmacol. Ther. 24:339–346 (1999).
P. A. Routledge, W. W. Stargel, B. B. Kitchell, A. Barchowsky, and D. G. Shand. Sex-related differences in the plasma protein binding of lignocaine and diazepam. Br. J. Clin. Pharmacol. 11:245–250 (1981).
M. Farrell, J. Ward, R. Mattick, W. Hall, G. V. Stimson, D. des Jarlais, M. Gossop, and J. Strang. Methadone maintenance treatment in opiate dependence: A review. Br. Med. J. 309:997–1001 (1994).
R. Fainsinger, T. Schoeller, and E. Bruera. Methadone in the management of cancer pain: a review. Pain 52:137–147 (1993).
C. Ripamonti, E. Zecca, and E. Bruera. An update on the clinical use of methadone for cancer pain. Pain 70:109–115 (1997).
K. R. Dyer, D. J. Foster, J. M. White, A. A. Somogyi, A. Menelaou, and F. Bochner. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin. Pharmacol. Ther. 65:685–694 (1999).
C. E. Inturrisi, W. A. Colburn, R. F. Kaiko, R. W. Houden, and K. M. Foley. Pharmacokinetics and pharmacodynamics of methadone in patients with chronic pain. Clin. Pharmacol. Ther. 41:392–401 (1987).
K. Wolff, A. Rostami-Hodjegan, S. Shires, A. W. M. Hay, M. Feely, R. Calvert, D. Raistrick, and G. T. Tucker. The pharmacokinetics of methadone in healthy subjects and opiate users. Br. J. Clin. Pharmacol. 44:325–334 (1997).
C. B. Eap, C. Cuendet, and P. Baumann. Binding of dmethadone, l-methadone and d-l-methadone to protein in plasma of healthy volunteers: Role of the variants of a1-acid glycoprotein. Clin. Pharmacol. Ther. 47:338–346 (1990).
E. Gomez, R. Martinez-Jorda, E. Suarez, M. J. Garrido, and R. Calvo. Altered methadone analgesia due to changes in plasma protein binding: Role of the route of administration. Gen. Pharmacol. 26:1273–1276 (1995).
D. Rostami-Hodjegan, W. Wolff, A. W. M. Hay, D. Raistrick, R. Calvert, and G. T. Tucker. Population pharmacokinetics of methadone in opiate users: Characterization of time-dependent changes. Br. J. Clin. Pharmacol. 48:43–52 (1999).
D. J. Foster, A. A. Somogyi, and F. Bochner. Methadone Ndemethylaction in human liver microsomes: Lack of stereoselectivity and involvement of CYP3A4. Br. J. Clin. Pharmacol. 47:403–412 (1999).
R. K. Verbeeck. J-A Cardinal, and S. M. Wallace. Effect of age and gender on the plasma binding of acidic and basic drugs. Eur. J. Clin. Pharmacol. 27:91–97 (1984).
R. E. Bartok and R. M. Craft. Sex differences in opioid antinociception. J. Pharmacol. Exp. Ther. 282:769–778 (1997).
J. Candido, K. Lutfy, B. Billings, V. Sierra, A. Duttaroy, C. E. Inturrisi, and B. C. Yoburn. Effect of adrenal and sex hormones on opioid analgesia and opioid receptor regulation. Pharmacol. Biochem. Behav. 42:685–692 (1992).
F. E. D'Amour and D. L. Smith. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 72:74–79 (1941).
K. Wolff, M. Sanderson, A. W. M. Hay, and D. Raistrick. Methadone concentrations in plasma and their relationship to drug dosage. Clin. Chem. 37:205–209 (1991).
M. J. Garrido, M. Valle, R. Calvo, and I. F. Troconiz. Altered plasma and brain disposition and pharmacodynamics of methadone in abstinent rats. J. Pharmacol. Exp. Ther. 288:179–187 (1999).
E. I. Ette, A. W. Kelman, C. A. Howie, and B. Whiting. Analysis of animal pharmacokinetic data: performance of the one point per animal design. J. Pharmacokinet. Biopharm. 23:551–566 (1995).
L. B. Sheiner, D. R. Stanski, S. Vozeh, R. D. Miller, and J. Ham. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 25:358–371 (1979).
A. M. Davis and C. E. Inturrisi. d-Methadone blocks morphine tolerance and N-methyl-d-aspartate induced hyperalgesia. J. Pharmacol Exp. Ther. 289:1048–1053 (1999).
K. L. Kepler, K. M. Standifer, D. Paul, G. W. Pasternak, B. Kest, and R. J. Bodnar. Gender effects upon central opioid analgesia. Pain 45:87–94 (1991).
R. M. Craft, J. A. Stratmann, R. E. Bartok, T. I. Walpole, and S. J. King. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology 143:1–7 (1999).
K. Wolff, A. Rostami-Hodjegan, A. W. M. Hay, D. Raistrick, and G. Tucker. Population based pharmacokinetic approach for methadone monitoring of opiate addicts: Potential clinical utility. Addiction 95:1771–1783 (2000).
G. S. F. Ling, J. C. Umans, and C. E. Inturrisi. Methadone: Radioimmunoassay and pharmacokinetics in the rat. J. Pharmacol. Exp. Ther. 217:147–151 (1981).
M.-I. Nilsson, E. Anggard, and J. Holmstrand. and L-M Gunne. Pharmacokinetics of methadone during maintenance treatment: Adaptive changes during the induction phase. Eur. J. Clin. Pharmacol. 22:343–349 (1982).
K. Wilson, C. N. Reynolds, and D. Burnett. Inter and intraindividual variation in the metabolism of methaqualone in man after a single oral dose. Eur. J. Clin. Pharmacol. 13:291–297 (1978).
J. D. Lane, J. F. Steege, S. L. Rupp, and C. M. Kuhn. Menstrual cycle effects on caffeine elimination in the human female. Eur. J. Clin. Pharmacol. 43:543–546 (1992).
M. Rowland and T. N. Tozer. Clinical Pharmacokinetics: Concepts and Applications 3rd Ed. William & Wilkins, Media, PA, 1995 pp. 137–155.
D. J. Greenblat, H. Friedman, E. S. Burstein, J. M. Scavone, G. T. Blyden, H. R. Ochs, L. G. Miller, J. S. Harmatz, and R. I. Shader. Trazodone kinetics: Effect of age, gender, and obesity. Clin. Pharmacol. Ther. 42:193–200 (1987).
E. Schwartz, R. S. Matteo, E. Ornstein, W. L. Young, and K. L. Myers. Pharmacokinetics of sufentanil in obese patients. Anesth. Analg. 73:790–793 (1991).
C. M. Hunt, W. R. Westerkam, and G. M. Stave. Effect of age and gender on the activity of human hepatic CYP3A. Biochem. Pharmacol. 44:275–283 (1992).
E. K. Krzanowska and R. J. Bodner. Morphine antinociception elicited from the ventrolateral periaductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 821:224–230 (1999).
J. S. Boyer, M. M. Morgan, and R. M. Craft. Microinjection of morphine into rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 796:315–318 (1998).
S. J. Liu, D. L. Roerig, and R. I. H. Wang. Brain and plasma levels of methadone and their relationship to analgesic activity of methadone in rats. Drug Metab. Dispos. 11:335–338 (1983).
K. Kristensen, C. B. Christensen, and L. L. Christrup. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 56:45–50 (1995).
K. Kristensen, T. Blemmer, H. R. Angelo, L. L. Christrup, N. E. Drenck, S. M. Rasmussen, and P. Sjogren. Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther. Drug Monitor. 18:221–227 (1996).
M. A. Carlos, P. du Souich, R. Carlos, E. Suarez, J. C. Lukas, and R. Calvo. Effect of omeprazole on oral and intravenous RS-methadone pharmacokinetics and pharmacodynamics in the rat. J. Pharm. Sci. 91:1627–1638 (2002).
C. Cordon-Cardo, J. P. O'Brien, D. Casals, L. Rittman-Grauer, J. L. Bierdler, M. R. Melamed, and J. R. Bertino. Multidrug resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA 86:695–698 (1989).
R. Bouer, L. Barthe, C. Philibert, C. Tourmaire, J. Woodley, and G. Houin. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: in vitro studies using the rat-inverted intestinal sack. Fundam. Clin. Pharmacol. 13:494–500 (1999).
S. J. Thopmson, D. V. M. Kari Koszdin, and C. M. Bernards. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology 92:1392–1399 (2000).
C. J. R. Parker, J. M. Hunter, and S. L. Snowdon. Effect of age, gender, and anesthetic technique on the pharmacodynamics of atracurium. Br. J. Anaesth. 70:38–41 (1993).
E. Sarton, E. Olofsen, R. Romberg, J. Hartigh, B. Kest, D. Nieuwenhuijs, A. Burm, L. Teppema, and A. Dahan. Sex differences in morphine analgesia. Anesthesiology 93:1245–1254 (2000).
R. P. Hammer. Mu-opiate receptor binding in the medial preoptic area is cyclical and sexually dimorphic. Brain Res. 515:187–192 (1990).
R. B. Simerly, L. Swanson, and R. A. Gorski. Demonstration of a sexual dimorphism in the distribution of serotonin inmunoreactive fevers in the medial preoptic nucleus of the rat. J. Comp. Neurol. 225:151–176 (1984).
K. L. Kepler, B. Kest, J. M. Kiefel, M. L. Cooper, and R. J. Bodnar. Roles of gender and gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol. Biochem. Behav. 34:119–127 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodriguez, M., Carlos, M.A., Ortega, I. et al. Sex Specificity in Methadone Analgesia in the Rat: A Population Pharmacokinetic and Pharmacodynamic Approach. Pharm Res 19, 858–867 (2002). https://doi.org/10.1023/A:1016117218760
Issue Date:
DOI: https://doi.org/10.1023/A:1016117218760