Abstract
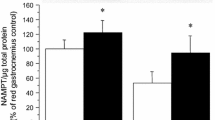
Since the glucose-lowering effects of vanadium could be related to increased muscle glycogen synthesis, we examined the in vivo effects of vanadium and insulin treatment on glycogen synthase (GS) activation in Zucker fatty rats. The GS fractional activity (GSFA), protein phosphatase-1 (PP1), and glycogen synthase kinase-3 (GSK-3) activity were determined in fatty and lean rats following treatment with bis(maltolato)oxovanadium(IV) (BMOV) for 3 weeks (0.2 mmol/kg/day) administered in drinking water. Skeletal muscle was freeze-clamped before or following an insulin injection (5 U/kg i.v.). In both lean and fatty rats, muscle GSFA was significantly increased at 15 min following insulin stimulation. Vanadium treatment resulted in decreased insulin levels and improved insulin sensitivity in the fatty rats. Interestingly, this treatment stimulated muscle GSFA by 2-fold (p < 0.05) and increased insulin-stimulated PP1 activity by 77% (p < 0.05) in the fatty rats as compared to untreated rats. Insulin resistance, vanadium and insulin in vivo treatment did not affect muscle GSK-3β activity in either fatty or lean rats. Therefore, an impaired insulin sensitivity in the Zucker fatty rats was improved following vanadium treatment, resulting in an enhanced muscle glucose metabolism through increased GS and insulin-stimulated PP1 activity.
Similar content being viewed by others
References
Vestergaard H, Lund S, Larsen FS, Bjerrum OJ, Pedersen O: Glycogen synthase and phosphofructokinase protein and mRNA levels in skeletal muscle from insulin-resistant patients with non-insulin-dependent diabetes mellitus. J Clin Invest 91: 2342–2350, 1993
Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB: Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3–kinase, in muscle in type 2 diabetes. J Clin Invest 104: 733–741, 1999
Damsbo P, Vaag A, Hother-Nielsen O, Beck-Nielsen H: Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 34: 239–245, 1991
Kahn CR: Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43: 1066–1084, 1994
Kida Y, Esposito-Del Puente A, Bogardus C, Mott DM: Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest 85: 476–481, 1990
Cohen P: In: P.D. Boyer, E.G. Krebs (eds). The Enzymes, Vol. 17. Academic Press, Orlando, FL, 1986, pp 461–479
Dent P, Lavoinne A, Nakielny S, Caudwell FB, Watt P, Cohen P: The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature 348: 302–308, 1990
Poulter L, Ang SG, Gibson BW, Williams DH, Holmes CF, Caudwell FB, Pitcher J, Cohen P: Analysis of the in vivo phosphorylation state of rabbit skeletal muscle glycogen synthase by fast-atom-bombardment mass spectrometry. Eur J Biochem 175: 497–510, 1988
Nakielny S, Campbell DG, Cohen P: The molecular mechanism by which adrenalin inhibits glycogen synthesis. Eur J Biochem 199: 713–722, 1991
Parker PJ, Caudwell FB, Cohen P: Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem 130: 227–234, 1983
Embi N, Rylatt DB, Cohen P: Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107: 519–527, 1980
Wang Y, Roach PJ: Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3. Dominant role of the phosphorylation of Ser-640 (site-3a). J Biol Chem 268: 23876–23880, 1993
Ramakrishna S, Benjamin WB: Insulin action rapidly decreases multifunctional protein kinase activity in rat adipose tissue. J Biol Chem 263: 12677–12681, 1988
Welsh GI, Proud CG: Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J 294: 625–629, 1993
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA: Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789, 1995
Murai H, Okazaki M, Kikuchi A: Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signaldependent inactivation. FEBS Lett 392: 153–160, 1996
Cross DA, Watt PW, Shaw M, van der Kaay J, Downes CP, Holder JC, Cohen P: Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett 406: 211–215, 1997
Alemany S, Pelech S, Brierley CH, Cohen P: The protein phosphatases involved in cellular regulation. Evidence that dephosphorylation of glycogen phosphorylase and glycogen synthase in the glycogen and microsomal fractions of rat liver are catalysed by the same enzyme: protein phosphatase-1. Eur J Biochem 156: 101–110, 1986
Lawrence JC Jr, Skurat AV, Roach PJ, Azpiazu I, Manchester J: Glycogen synthase: Activation by insulin and effect of transgenic overexpression in skeletal muscle. Biochem Soc Trans 25: 14–19, 1997
Ragolia L, Begum N: Protein phosphatase-1 and insulin action. Mol Cell Biochem 182: 49–58, 1998
Kida Y, Raz I, Maeda R, Nyomba BL, Stone K, Bogardus C, Sommercorn J, Mott DM: Defective insulin response of phosphorylase phosphatase in insulin-resistant humans. J Clin Invest 89: 610–617, 1992
Srinivasan M, Patel MS: Glycogen synthase activation in the epididymal adipose tissue from chronic hyperinsulinemic/obese rats. J Nutr Biochem 9: 81–87, 1998
Ortmeyer HK: Insulin increases liver protein phosphatase-1 and protein phosphatase-2C activities in lean, young adult rhesus monkeys. Horm Metab Res 30: 705–710, 1998
Heyliger CE, Tahiliani AG, McNeill JH: Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science 227: 1474–1477, 1985
Meyerovitch J, Farfel Z, Sack J, Shechter Y: Oral administration of vanadate normalizes blood glucose levels in streptozotocintreated rats. Characterization and mode of action. J Biol Chem 262: 6658–6662, 1987
Brichard SM, Pottier AM, Henquin JC: Long term improvement of glucose homeostasis by vanadate in obese hyperinsulinemic fa/fa rats. Endocrinology 125: 2510–2516, 1989
Meyerovitch J, Rothenberg P, Shechter Y, Bonner-Weir S, Kahn CR: Vanadate normalizes hyperglycemia in two mouse models of non-insulin-dependent diabetes mellitus. J Clin Invest 87: 1286–1294, 1991
Yuen VG, Vera E, Battell ML, Li WM, McNeill JH: Acute and chronic oral administration of bis(maltolato)oxovanadium(IV) in Zucker diabetic fatty (ZDF) rats. Diabetes Res Clin Pract 43: 9–19, 1999
Goldfine AB, Simonson DC, Folli F, Patti ME, Kahn CR: Metabolic effects of sodium metavanadate in humans with insulin-dependent and noninsulin-dependent diabetes mellitus in vivo and in vitro studies. J Clin Endocrinol Metab 80: 3311–3320, 1995
Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L: Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 95: 2501–2509, 1995
Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999
Gregory JS, Boulton TG, Sang BC, Cobb MH: An insulin-stimulated ribosomal protein S6 kinase from rabbit liver. J Biol Chem 264: 18397–18401, 1989
Pelech SL, Krebs EG: Mitogen-activated S6 kinase is stimulated via protein kinase C-dependent and independent pathways in Swiss 3T3 cells. J Biol Chem 262: 11598–11606, 1987
Semiz S, Orvig C, McNeill JH: Effects of diabetes, vanadium, and insulin on glycogen synthase activation in Wistar rats. Mol Cell Biochem 231: 23–25, 2002
Bhanot S, Salh BS, Verma S, McNeill JH, Pelech SL: In vivo regulation of protein-serine kinases by insulin in skeletal muscle of fructose-hypertensive rats. Am J Physiol 277: E299–307, 1999
Ortmeyer HK, Bodkin NL, Hansen BC: Insulin-mediated glycogen synthase activity in muscle of spontaneously insulin-resistant and diabetic rhesus monkeys. Am J Physiol 265: R552–558, 1993
Foulkes JG, Jefferson LS: Protein phosphatase-1 and-2A activities in heart, liver, and skeletal muscle extracts from control and diabetic rats. Diabetes 33: 576–579, 1984
Cohen P, Klumpp S, Schelling DL: An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett 250: 596–600, 1989
Beck-Nielsen H: General characteristics of the insulin resistance syndrome: Prevalence and heritability. European Group for the study of Insulin Resistance (EGIR). Drugs 58: 7–10; discussion 75-82, 1999
Bruce CR, Lee JS, Hawley JA: Postexercise muscle glycogen resynthesis in obese insulin-resistant Zucker rats. J Appl Physiol 91: 1512–1519, 2001
Beck-Nielsen H, Vaag A, Damsbo P, Handberg A, Nielsen OH, Henriksen JE, Thye-Ronn P: Insulin resistance in skeletal muscles in patients with NIDDM. Diabetes Care 15: 418–429, 1992
Lavoie L, Bollen M, Stalmans W, van de Werve G: Increased synthase phosphatase activity is responsible for the super-activation of glycogen synthase in hepatocytes from fasted obese Zucker rats. Endocrinology 129: 2674–2678, 1991
Villar-Palasi C: Oligo-and polysaccharide inhibition of muscle transferase D phosphatase. Ann NY Acad Sci 166: 719–730, 1969
Mellgren RL, Coulson M: Coordinated feedback regulation of muscle glycogen metabolism: Inhibition of purified phosphorylase phosphatase by glycogen. Biochem Biophys Res Commun 114: 148–154, 1983
Laurent D, Hundal RS, Dresner A, Price TB, Vogel SM, Petersen KF, Shulman GI: Mechanism of muscle glycogen autoregulation in humans. Am J Physiol Endocrinol Metab 278: E663–668, 2000
Suzuki Y, Lanner C, Kim JH, Vilardo PG, Zhang H, Yang J, Cooper LD, Steele M, Kennedy A, Bock CB, Scrimgeour A, Lawrence JC Jr, DePaoli-Roach AA: Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol Cell Biol 21: 2683–2694, 2001
Borthwick AC, Wells AM, Rochford JJ, Hurel SJ, Turnbull DM, Yeaman SJ: Inhibition of glycogen synthase kinase-3 by insulin in cultured human skeletal muscle myoblasts. Biochem Biophys Res Commun 210: 738–745, 1995
Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG: Increased glycogen synthase kinase-3 activity in diabetes-and obesity-prone C57BL/6J mice. Diabetes 48: 1662–1666, 1999
Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR: Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 49: 263–271, 2000
Singh LP, Crook ED: The effects of glucose and the hexosamine biosynthesis pathway on glycogen synthase kinase-3 and other protein kinases that regulate glycogen synthase activity. J Investig Med 48: 251–258, 2000
Sung CK, Choi WS, Scalia P: Insulin-stimulated glycogen synthesis in cultured hepatoma cells: Differential effects of inhibitors of insulin signaling molecules. J Recept Signal Transduct Res 18: 243–263, 1998
Skurat AV, Roach PJ: Phosphorylation of sites 3a and 3b (Ser640 and Ser644) in the control of rabbit muscle glycogen synthase. J Biol Chem 270: 12491–12497, 1995
Skurat AV, Roach PJ: Multiple mechanisms for the phosphorylation of C-terminal regulatory sites in rabbit muscle glycogen synthase expressed in COS cells. Biochem J 313: 45–50, 1996
Skurat AV, Dietrich AD, Roach PJ: Glycogen synthase sensitivity to insulin and glucose-6–phosphate is mediated by both NH2–and COOH-terminal phosphorylation sites. Diabetes 49: 1096–1100, 2000
Pugazhenthi S, Khandelwal RL: Regulation of glycogen synthase activation in isolated hepatocytes. Mol Cell Biochem 149-150: 95–101, 1995
Chalfant CE, Ciaraldi TP, Watson JE, Nikoulina S, Henry RR, Cooper DR: Protein kinase Ctheta expression is increased upon differentiation of human skeletal muscle cells: Dysregulation in type 2 diabetic patients and a possible role for protein kinase Ctheta in insulin-stimulated glycogen synthase activity. Endocrinology 141: 2773–2778, 2000
Halberstam M, Cohen N, Shlimovich P, Rossetti L, Shamoon H: Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes 45: 659–666, 1996
Cam MC, Cros GH, Serrano JJ, Lazaro R, McNeill JH: In vivo antidiabetic actions of naglivan, an organic vanadyl compound in streptozotocin-induced diabetes. Diabetes Res Clin Pract 20: 111–121, 1993
Goldfine AB, Patti ME, Zuberi L, Goldstein BJ, LeBlanc R, Landaker EJ, Jiang ZY, Willsky GR, Kahn CR: Metabolic effects of vanadyl sulfate in humans with non-insulin-dependent diabetes mellitus: in vivo and in vitro studies. Metabolism 49: 400–410, 2000
Cam MC, Brownsey RW, McNeill JH: Mechanisms of vanadium action: insulin-mimetic or insulin-enhancing agent? Can J Physiol Pharmacol 78: 829–847, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Semiz, S., McNeill, J.H. Oral treatment with vanadium of Zucker fatty rats activates muscle glycogen synthesis and insulin-stimulated protein phosphatase-1 activity. Mol Cell Biochem 236, 123–131 (2002). https://doi.org/10.1023/A:1016116700632
Issue Date:
DOI: https://doi.org/10.1023/A:1016116700632