Abstract
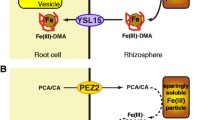
Iron is abundant in most soils, but ferric compounds are almost insoluble. Therefore, plant roots use as tools acidification and enzymatic reduction of iron at the outer cell surface (strategy I) or solubilization by phytosiderophores, which are specific ferric chelators (strategy II). In the first case, iron is taken up as Fe2+ into the root symplast, and in the latter one, iron is taken up as Fe(III) complex. The path of iron from the root surface, up to the point of the xylem vessels within the central cylinder, may be completely symplasmic. However, a part of this route also may be an apoplasmic one, through the free space of the cell walls of rhizodermis and cortex (apoplast). In the endodermis, the Casparian band forms a strict barrier for apoplasmic transport; to move past this site, all ions must enter the symplast. During symplasmic transport, the intracellular environment is protected against the reactive species of iron by handling of iron in chelated forms. A promising candidate for this purpose is the plant-endogenous chelator nicotianamine. At the apoplasmic site, iron can be oxidized followed by precipitation as hydroxide or phosphate compounds. Thus, a pool of apoplastic iron can be formed, as shown by reductive mobilization or by proton-induced X-ray emission. This pool may be remobilized when iron deficiency takes place. During radial transport to the vessels, vacuoles may compete with the transport stream forming an iron store. When there is an iron excess, as in plants growing in waterlogged soils or by experimental techniques, plants can escape the deleterious effects of free iron by depositing it in phytoferritin, a storage protein inducible under iron excess. Also, nicotianamine forms a pool of metabolically available iron. Thus, in roots cells of the nicotianamine-free tomato mutant chloronerva iron precipitations occur as evidenced by energy dispersive X-ray analysis and the electron microscopic energy loss technique of energy spectroscopic imaging. Future research concerning the plant root's iron metabolism are needed to clarify the function of nicotianamine in intra- and intercellular iron trafficking and to identify the so-called iron-sensor which mediates the regulation of iron acquisition reactions of rhizodermal cells in response to the iron nutritional status of the plant.
Similar content being viewed by others
References
Becker R, Fritz E and Manteuffel R 1995 Subcellular localization and characterization of excessive iron in the nicotianamine-less tomato mutant chloronerva. Plant Physiol. 108, 269-275.
Bienfait H F, De Weger L A and Kramer D 1987 Control of the development of iron-efficiency reactions in potato as a response to iron deficiency is located in the roots. Plant Physiol. 83, 244-247.
Bienfait H F, Van den Briel W and Mesland-Mul N T 1985 Free space iron pools in roots. Generation and mobilization. Plant Physiol. 78, 596-600.
Böhme H and Scholz G 1960 Versuche zur Normalisierung des Phänotyps der Mutante chloronerva von Lycopersicon esculentum Mill. Kulturpflanze 8, 93-109.
Boyer R F, Clark H M and Sanchez S 1989 Solubilization of ferrihydrite iron by plant phenolics: A model for rhizosphere processes. J. Plant Nutr. 12, 581-592.
Briat J F and Lobréaux S 1997 Iron transport and storage in plants. Trends Plant Sci. 2, 187-193.
Brown J C, Ambler J E, Chaney R L and Foy C D 1972 Differential responses of plant genotypes of micronutrients. In Micronutrients in Agriculture. Eds. J J Mortvedt, P M Giordano and W L Lindsay. pp 385-418. Soil Sci. Am., Madison, WI.
Buděšínský M, Budzikiewicz H, Procházka Ž, Ripperger H, Römer A, Scholz G and Schreiber K 1980 Nicotianamine, a possible phytosiderophore of general occurrence. Phytochemistry 19, 2295-2297.
DeBoer A H, Prius H B A and Zanstra P E 1983 Biphasic composition of transroot electrical potential in roots of Plantago species: Involvement of spacially separated electrogenic pumps. Planta 157, 259-266.
Dykema P E, Sipes P R, Marie A, Biermann B J, Crowell D N and Randall S K 1999 A new class of proteins capable of binding transition metals. Plant Mol. Biol. 41, 139-150.
Fox T C and Guerinot M L 1998 Molecular biology of cation transport in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 669-696.
Gerendás J and Schurr U 1999 Physicochemical aspects of ion relations and pH regulation in plants-a quantitative approach. J. Exp. Bot. 50, 1101-1114.
Gottschalk W G 1987 Improvement of the selection value of gene dgl through recombination. Pisum Newslett. 19, 9-11.
Grill E, Winnacker E L and Zenk M H 1985 Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 230, 674-676.
Gris E 1843 Mémoir relatif à l'action des composés solubles ferrugineux sur la végétation. Compt. Rend. Acad. Sci. (Paris) 17, 679.
Grusak M A and Pezeshgi S 1996 Shoot-to-root signal transmission regulates root Fe(III) reductase activity in the dgl mutant of pea. Plant Physiol. 110, 329-334.
Grusak MA, Welch R Mand Kochian L V 1990 Physiological characterization of a single-gene mutant of Pisum sativum exhibiting excess iron accumulation. I. Root iron reduction and iron uptake. Plant Physiol. 93, 976-981.
Guerinot M L and Yi Y 1994 Iron-nutritious, noxious, and not readily available. Plant Physiol. 104, 815-820.
Helder R J and Boerma J 1969 An electron-microscopical study of the plasmodesmata in the roots of young barley seedlings. Acta Bot. Neerl. 18, 99-107.
Higuchi K, Kanazawa K, Nishizawa N K and Mori S 1996 The role of nicotianamine synthase in response to Fe nutrition status in Gramineae. Plant Soil 178, 171-177.
Jooste J H and De Bruyn J A 1979 The dual mechanism of iron absorption in bean root and leaf tissues. Jl. S. Afr. Bot. 45, 243-248.
Kneen B E, LaRue T A, Welch R M and Weeden N F 1990 Pleiotropic effects of brz. A mutation in Pisum sativum (L.) cv. 'Sparkle' conditioning decreased nodulation and increased iron uptake and leaf necrosis. Plant Physiol. 93, 717-722.
Köhler B and Raschke K 2000 The delivery of salts to the xylem. Three types of anion conductance in the plasmalemma of the xylem parenchyma of roots of barley. Plant Physiol. 122, 243-254.
Landsberg E C 1986 Function of rhizodermal transfer cells in the Fe stress response mechanism of Capsicum annuum L. Plant Physiol. 82, 511-517.
Liu D H, Adler K and Stephan U W 1998 Iron-containing particles accumulate in organelles and vacuoles of leaf and root cells in the nicotianamine-free tomato mutant chloronerva. Protoplasma 201, 213-220.
Lobréaux S, Massenet O and Briat J F 1992 Iron induces ferritin synthesis in maize plantlets. Plant Mol. Biol. 19, 563-575.
Longnecker N and Welch R M 1990 Accumulation of apoplastic iron in plant roots. Plant Physiol. 92, 17-22.
López-Millán A F, Morales F, Abadía A and Abadía J 2000 Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiol. 124, 873-884.
Ma J F, Kusano G, Kimura S and Nomoto K 1993 Specific recognition of mugineic acid-ferric complex by barley roots. Phytochemistry 34, 599-603.
Maas F M, Van de Wetering D A M, Van Beusichem M L and Bienfait H F 1988 Characterization of phloem iron and its possible role in the regulation of Fe-efficiency reactions
Marentes E, Stephens B W and Grusak M A 1997 Characterization of a phloem mobile chelator involved in the phloem transport of iron from vegetative tissues to developing seeds of pea. Abstr. 9th Int. Symp. Iron Nutr. Interact. Plants, Stuttgart/Germany July 20-25 1997, p. 74.
Marschner H 1995 Mineral Nutrition of Higher Plants. Academic Press, New York. 889 p.
Marschner H, Römheld V and Ossenberg-Neuhaus H 1982 Rapid method for measuring changes in pH and reducing processes along roots of intact plants. Zschr. Pflanzenphysiol. 105, 407-416.
Miller G W, Hasegawa S, Shigematsu A and Welkie G W 1994 Mechanisms of iron uptake from rhodotorulate iron by tomato. In Biochemistry of Metal Micronutrients in the Rhizosphere. Eds J A Manthey, D E Crowley and D G Luster. pp 267-284. Lewis Publishers, Boca Raton.
Newcomb E H 1967 Fine structure of protein-storing plastids in bean root tips. J. Cell Biol. 33, 143-163.
Olsen R A, Clark R B and Bennett J H 1981 The enhancement of soil fertility by plant roots. Amer. Scientist 69, 378-385.
Pich A, Hillmer S, Manteuffel R and Scholz G 1997 First immunohistochemical localization of the endogenous Fe2+-chelator nicotianamine. J. Exp. Bot. 48, 759-767.
Pich A, Scholz G and Stephan U W 1994 Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes. Nicotianamine as possible copper translocator. Plant Soil 165, 189-196.
Rauser W E 1990 Phytochelatins. Annu. Rev. Biochem. 59, 61-86.
Römheld V 1987 Different strategies for iron acquisition in higher plants. Physiol. Plant. 70, 231-234.
Römheld V and Marschner H 1983 Mechanism of iron uptake by peanut plants. I. FeIII reduction, chelate splitting, and release of phenolics. Plant Physiol. 71, 949-954.
Schmidke I, Krüger C, Frömmichen R, Scholz G and Stephan U W 1999 Phloem loading and transport characteristics of iron in interaction with plant-endogenous ligands in castor bean seedlings. Physiol. Plant. 106, 82-89.
Schmidt W 1999 Mechanism and regulation of reduction-based iron uptake in plants. New Phytol. 141, 1-26.
Seckbach J 1969 Iron content and ferritin in leaves of iron treated Xanthium pennsylvanicum plants: localization and diversity in the organelle. J. Ultrastruct. Res. 39, 65-76.
Shone M G T and Flood A V 1985 Measurement of the free space and sorption of large molecules by cereal roots. Plant Cell Environ. 8, 309-315.
Stephan U W and Grün M 1989 Physiological disorders of the nicotianamine-auxothroph tomato mutant chloronerva at different levels of iron nutrition. II. Iron deficiency response and heavy metal metabolism. Biochem. Physiol. Pflanzen 185, 189-200.
Stephan U W, Schmidke I, Stephan V W and Scholz G 1996 The nicotianamine molecule is made-to-measure for complexation of metal micronutrients in plants. BioMetals 9, 84-90.
Stephan U W and Scholz G 1990 Nicotianamine concentrations in iron sufficient and iron deficient sunflower and barley roots. J. Plant Physiol. 136, 631-634.
Strasser O, Köhl K and Römheld V 1999 Overestimation of apoplastic Fe in roots of soil grown plants. Plant Soil 210, 79-187.
Takagi S I 1976 Naturally occurring iron-chelating compounds in oat-and rice-root washings. I. Activity measurement and preliminary characterization. Soil Sci. Plant Nutr. 22, 423-433.
Theil E C 1987 Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 56, 289-315.
Tiffin L O 1966a Iron translocation. I. Plant culture, exudate sampling, iron-citrate analysis. Plant Physiol. 41, 510-514.
Tiffin L O 1966b Iron translocation. II. Citrate/iron ratios in plant stem exudates. Plant Physiol. 41, 515-518.
Von Wirén N, Klair S, Bansal S, Briat J F, Khodr H, Shioiri T, Leigh R A and Hider R C 1999 Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol. 119, 1107-1114.
Wang H L, Stephens B W and Grusak M A 1999 Identification of metal-chelating proteins and peptides in phloem sap. Abstr. Am. Soc. Plant Physiol. Ann. Meet. 24-28.7. 1999, p. 105.
Welch R M and LaRue T A 1990 Physiological characteristics of Fe accumulation in the 'Bronze' mutant of Pisum sativum L., cv. 'Sparkle' E 107 (brz brz). Plant Physiol. 93, 723-729.
Yehuda Z, Shenker M, Römheld V, Marschner H, Hadar Y and Chen Y 1996 The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiol. 112, 1273-1280.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stephan, U.W. Intra- and intercellular iron trafficking and subcellular compartmentation within roots. Plant and Soil 241, 19–25 (2002). https://doi.org/10.1023/A:1016086608846
Issue Date:
DOI: https://doi.org/10.1023/A:1016086608846