Abstract
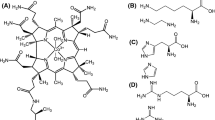
Purpose. To clarify the mechanism of covalent binding between human serum albumin (HSA) and drugs containing thiol groups, we studied the interactions between HSA and bucillamine (BA) and its derivatives.
Methods. To determine the concentration of HSA-drug conjugate, we used columns of N-methylpyridium polymer cross-linked with ethylene glycol dimethacrylate (4VP-Me), and analyzed the reaction between HSA and B A derivatives kinetically. Following pseudo first-order reaction kinetics, the rate constants of reduction of non-mercaptoalbumin (HNA) to mercaptoalbumin (HMA) (ka) and formation of HSA-drug conjugate (kc) were determined.
Results. Formation of HSA-drug conjugate was observed only for drugs containing one thiol group. In compound IV, the plots of ka and kc against pH were found to be linear. The HSA-drug conjugate was affected by various factors such as pKa, pH, temparture and the microenviroment of Cys34. The increases in ka and kc. against pH were mainly due to the increase in mercaptide ion concentration. Further, fatty acid affected the microenviroment of Cys34, which increased HSA-drug formation.
Conclusions. Cys34 located in a crevice on the surface of the protein plays an important role on the formation of HSA-drug conjugate. These results may be useful for elucidating the reaction mechanisms between various proteins and thiol compounds.
Similar content being viewed by others
REFERENCES
Era S, Serum Albumin: Recent progress in the understanding of its structure and pathophysiology. Acta Sch Med Univ Gifu 39:6–24, 1991.
Wallevik K, SS-interchanged and oxidized isomers of bovine serum albumin separated by isoelectric focusing. Biochim Biophys Acta 420:42–56, 1976.
Lewis S, Misra D and Shafer J, Determination of interactive thiol ionization in bovine serum albumin, glutathione, and other thiols by potentiometric difference titration. Biochemistry 19:6129–37, 1980.
Meyer D, Kramer H, Özer N, Brain C and Ketterer B, Kinetics and equilibria of S-nitrosothiol-thiol exchange between glutathione, penicillamines and serum albumin. FEBS Lett 345:177–80, 1994.
Joyce D, In vitro mechanism of oxidation of d-penicillamine in plasma. J Pharm Sci 80:289–91, 1991.
Narazaki R, Harada K, Sugii A and Otagiri M, Kinetic analysis for the covalent binding captopril to human serum albumin. J Pharm Sci submitted for publication.
Horiuchi M, Takashina H, Iwatani T and Iso T, Study on metapbolism of dithiol compounds. I. Isolation and identification of metabolites of N-(2-mercapto-2-methylpropanoyl)-L-cysteine (SA96) in blood and urine of rat. Yakugaku Zasshi 105:665–70, 1985.
Yeung J, Breckenridge A and Park B, Drug protein conjugate-IV: the effect of accute renal failure on the disposition of [14C]captopril in the rat. Biochem Pharmacol 32:2467–72, 1983.
Park B and Kitteringham N, Drug-protein conjugation and its immunological consequences. Drug Metab Rev 22:87–144, 1990.
Chen R, Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem 242:173–81, 1967.
Fava A, Iliceto A and Camera E, Kinetics of the thiol-disulfide exchange. J Am Chem Soc 79:833–8, 1957.
Carter D and Ho J, Structure of serum albumin. Adv Protein Chem 45:153–203, 1994.
Jocelyn P, The standard redox potential of cystein-cystine from the thiol-disulfide exchange reaction with glutathione and lipoic acid. Eur J Biochem 2:327–31, 1967.
Takabayashi K, Imada T, Saito Y and Inada Y, Coupling between fatty acid binding and sulfhydryl oxidation in bovine serum albumin. Eur J Biochem 136:291–5, 1983.
Hamilton A, Era S, Bhamidipati S and Reed R, Location of the three primary binding sites for long-chain fatty acid on bovine serum albumin. Proc Natl Acad Sci USA 88:2051–4, 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Narazaki, R., Hamada, M., Harada, K. et al. Covalent Binding Between Bucillamine Derivatives and Human Serum Albumin. Pharm Res 13, 1317–1321 (1996). https://doi.org/10.1023/A:1016057513490
Issue Date:
DOI: https://doi.org/10.1023/A:1016057513490