Abstract
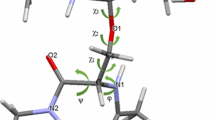
The disaccharide α-Kdo-(2→8)-α-Kdo (Kdo: 3-deoxy-d-manno-oct-2-ulosonic acid) represents a genus-specific epitope of the lipopolysaccharide of the obligate intracellular human pathogen Chlamydia. The conformation of the synthetically derived disaccharide α-Kdo-(2→8)-α-Kdo-(2→O)-allyl was studied in aqueous solution, and complexed to a monoclonal antibody S25-2. Various NMR experiments based on the detection of NOEs (or transfer NOEs) and ROEs (or transfer ROEs) were performed. A major problem was the extensive overlap of almost all 1H NMR signals of α-Kdo-(2→8)-α-Kdo-(2→O)-allyl. To overcome this difficulty, HMQC-NOESY and HMQC-trNOESY experiments were employed. Spin diffusion effects were identified using trROESY experiments, QUIET-trNOESY experiments and MINSY experiments. It was found that protein protons contribute to the observed spin diffusion effects. At 800 MHz, intermolecular trNOEs were observed between ligand protons and aromatic protons in the antibody binding site. From NMR experiments and Metropolis Monte Carlo simulations, it was concluded that α-Kdo-(2→8)-α-Kdo-(2→O)-allyl in aqueous solution exists as a complex conformational mixture. Upon binding to the monoclonal antibody S25-2, only a limited range of conformations is available to α-Kdo-(2→8)-α-Kdo-(2→O)-allyl. These possible bound conformations were derived from a distance geometry analysis using transfer NOEs as experimental constraints. It is clear that a conformation is selected which lies within a part of the conformational space that is highly populated in solution. This conformational space also includes the conformation found in the crystal structure. Our results provide a basis for modeling studies of the antibody–disaccharide complex.
Similar content being viewed by others
References
Arepalli, S.R., Glaudemans, C.P.J., Daves, G.D., Kovac, P. and Bax, A. (1995) J. Magn. Reson., B106, 195–198.
Asensio, J.L., Cañada, F.J. and Jimenez-Barbero, J. (1995) Eur. J. Biochem., 233, 618–630.
Bock, K., Thomsen, J.U., Kosma, P., Christian, R., Holst, O. and Brade, H. (1992) Carbohydr. Res., 229, 213–224.
Brade, H., Brabetz, W., Brade, L., Holst, O., Löbau, S., Lucakova, M., Mamat, U., Rozalski, A., Zych, K. and Kosma, P. (1997) J. Endotoxin Res., 4, 67–84.
Emsley, L. and Bodenhausen, G. (1992) J. Magn. Reson., 97, 135–148.
Fu, Y., Baumann, M., Kosma, P., Brade, L. and Brade, H. (1992) Infect. Immun., 60, 1314–1321.
Hwang, T.-L. and Shaka, A.J. (1992) J. Am. Chem. Soc., 114, 3157–3159.
Kosma, P., Bahnmüller, R., Schulz, G. and Brade, H. (1990) Carbohydr. Res., 208, 37–50.
Lian, L. Y., Barsukov, I. L., Sutcliffe, M. J., Sze, K. H. and Roberts, G. C. K. (1994) Methods Enzymol., 239, 657–700.
Massefski, W. and Redfield, A.G. (1988) J. Magn. Reson., 78, 150–155.
Mikol, V., Kosma, P. and Brade, H. (1994) Carbohydr. Res., 263, 35–42.
Moseley, H.N.B., Lee, W., Arrowsmith, C.H. and Krishna, N.R. (1997) Biochemistry, 36, 5239–5299.
Ni, F. (1994) Prog. NMR Spectrosc., 26, 517–606.
Peters, T., Meyer, B., Stuike-Prill, R., Somorjai, R. and Brisson, J.-R. (1993) Carbohydr. Res., 238, 49–73.
Peters, T. and Pinto, B.M. (1996) Curr. Opin. Struct. Biol., 6, 710–720.
Scheffler, K., Ernst, B., Katopodis, A., Magnani, J. L., Wang, W.T., Weisemann, R. and Peters, T. (1995) Angew. Chem., Int. Ed. Engl., 34, 1841–1844.
Scherf, T. and Anglister, J. (1993) Biophys. J., 64, 754–761.
Stuike-Prill, R. and Meyer, B. (1991) Eur. J. Biochem., 194, 903–919.
Vincent, S.J.F., Zwahlen, C., Post, C.B., Burgner, J.W. and Bodenhausen, G. (1997) Proc. Natl. Acad. Sci. USA, 94, 4383–4388.
Weimar, T., Harris, S. L., Pitner, J. B., Bock, K. and Pinto, B. M. (1995) Biochemistry, 34, 13672–13680.
Weimar, T., Imberty, A., Perez, S. and Peters, T. (1997) Theochem., 395/396, 297–311.
Zwahlen, C., Vincent, S.J.F., Di Bari, L., Levitt, M.H. and Bodenhausen, G. (1994) J. Am. Chem. Soc., 116, 362–368.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sokolowski, T., Haselhorst, T., Scheffler, K. et al. Conformational analysis of a Chlamydia-specific disaccharide α-Kdo-(2→8)-α-Kdo-(2→O)-allyl in aqueous solution and bound to a monoclonal antibody: Observation of intermolecular transfer NOEs. J Biomol NMR 12, 123–133 (1998). https://doi.org/10.1023/A:1016047602190
Issue Date:
DOI: https://doi.org/10.1023/A:1016047602190