Abstract
Purpose. To develop and characterize a biodegradable polymeric sustained release surgical paste formulation for taxol.
Methods. Taxol was incorporated into poly(ε-caprolactone) (PCL) or blends of PCL with methoxypolyethylene glycol, MW 350 (MePEG). The surgical pastes were characterized using gel permeation chromatography, thermal analysis, scanning electron microscopy, and a tensile strength tester. In vitro release data for taxol from the surgical paste formulations was carried out at 37°C in phosphate buffered saline, pH 7.4, using an HPLC assay for taxol. Antiangiogenic activity of the formulations were assessed using a chick chorioallantoic membrane assay (CAM).
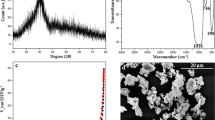
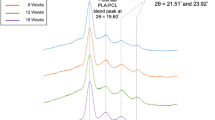
Results. The addition of up to 30% MePEG in PCL decreased the melting point of PCL by 5°C and the tensile strength by 152.7 N/cm2 to 26.7 N/cm2 but increased the degree of PCL crystallinity from 42% to 51%. Taxol showed a biphasic in vitro release profile composed of a burst phase lasting 1 or 2 days followed by a period of slow sustained drug release. There was no significant difference in the release profiles of taxol from two different sources of PCL. The addition of MePEG increased the amount of water taken up by the polymer blends but decreased the rate of taxol release. The formulations were shown to have antiangiogenic activity by the CAM assay at levels as low as 0.1% taxol using 3 mg surgical paste pellets.
Conclusions. Our surgical paste formulations for taxol give sustained release while having physical properties which can be adjusted using additives.
Similar content being viewed by others
REFERENCES
C. M. Spencer and D. Faulds. Paclitaxel: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 48:794–847 (1994).
N. Onetto, R. Canetta, B. Winograd, R. Catane, M. Dougan, J. Grechko, J. Burroughs and M. Rozencweig. Overview of taxol safety. Journal of the National Cancer Institute Monographs. 15:131–139 (1993).
A. M. C. Oktaba, W. L. Hunter and A. L. Arsenault. Taxol: a potent inhibitor of angiogenesis and tumor angiogenesis. Submitted to International Journal of Cancer. (1995).
J. Folkman and M. Klagsburg. Angiogenic factors. Science. 235:442–447 (1987).
M. E. Stearns and M. Wang. Taxol blocks processes essential for prostate tumour cell (PC-3 ML) invasion and metastases. Cancer Research. 52:3776–3781 (1992).
V. T. Devita, Jr., S. Hellman and S. A. Rosenberg, (eds.) Cancer: Principles and Practice of Oncology. 3rd ed. Philadelphia: J. B. Lippincott Co., 1989.
F. H. Hochberg and A. Pruitt. Assumptions in the radiotherapy of glioblastoma. Neurology. 30:907–911 (1980).
K. E. Wallner, J. H. Galicich, G. Krol, E. Arbit and M. G. Malkin. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. International Journal of Radiation Oncology Biology and Physics. 16:1405–1409 (1988).
C. G. Pitt. Poly-ε-caprolactone and its copolymers. In M. Chasinand R. Langer eds. Biodegradable Polymers as Drug Delivery Systems, Marcel Dekker: New York, 1990, pp. 71–120.
C. G. Pitt, M. M. Gratzl, A. R. Jeffcoat, R. Zweidinger and A. Schindler. Sustained drug delivery ststems II: factors affecting release rates from poly(ε-caprolactone) and related biodegradable polyesters. Journal of Pharmaceutical Sciences. 68:1534–1538 (1979).
G. Perego, G. D. Cella, N. N. Aldini, M. Fini and R. Giardino. Preparation of a new nerve guide from a poly(L-lactide-co-6-caprolactone). Biomaterials. 15:189–193 (1994).
P. Bruin, J. Smedinga, A. J. Pennings and M. F. Jonkman. Biodegradable lysine diisocyanate-based poly(glycolide-co-ε-caprolactone)-urethane network in artificial skin. Biomaterials. 11:291–295 (1990).
C. G. Pitt, M. M. Gratzl, G. L. Kimmel, J. Surles and A. Schindler. Aliphatic polyesters II. The degradation of poly(DL-lactide), poly(ε-caprolactone), and their copolymers in vivo. Biomaterials. 2:215–220 (1981).
S. C. Woodward, P. S. Brewer, F. Moatamed, A. Schindler and C. G. Pitt. The intracellular degradation of poly(ε-caprolactone). Journal of Biomedical Materials Research. 19:437–444 (1985).
O. F. Solomon and I. Z. Ciuta. Détermination di la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité. Journal of Applied Polymer Science. 6:683–686 (1962).
J. D. Dugan, Jr., M. T. Lawton, B. Glaser and H. Brem. A new technique for explantation and in vitro cultivation of chicken embryos. The Anatomical Record. 229:125–128 (1991).
S. L. Rosen. Fundamental Principles of Polymeric Materials. (Second Edition) John Wiley & Sons, Inc., New York, 1993.
J. M. G. Cowie. Polymers: Chemistry & Physics of Modern Materials. International Textbook Company Limited, Aylesbury, 1973.
J. D. Adams, K. P. Flora, B. R. Goldspiel, J. W. Wilson, S. G. Arbuck and R. Finley. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. Journal of the National Cancer Institute Monographs. 15:141–147 (1993).
R. Bawa, R. A. Siegel, B. Marasca, M. Karel and R. Langer. An explanation for the controlled release of macromolecules from polymers. Journal of Controlled Release. 1:259–267 (1985).
R. A. Siegel and R. Langer. Mechanistic studies of macromolecular drug release from macroporous polymers. II. Models for the slow kinetics of drug release. Journal of Controlled Release. 14:153–167 (1990).
C. Sturesson, J. Carlfors, K. Edsman and M. Andersson. Preparation of biodegradable poly(lactic-co-glycolic) acid microspheres and their in vitro release of timolol maleate. International Journal of Pharmaceutics. 89:235–244 (1993).
J. Folkman. Toward an understanding of angiogenesis: search and discovery. Perspectives in Biology and Medicine. 29:10–36 (1985).
R. Steiner. Angiostatic activity of anticancer agents in the chick embryo chorioallantoic membrane (CHE-CAM) assay. In R. Steiner, P. B. Weiszand R. Langer eds. Angiogenesis, Birkhauser Verlag: Berlin, 1992, pp. 449–454.
O. Kubo, Y. Tajika, Y. Muragaki, H. Hiyama, K. Takakura, M. Yoshida and M. Kumakura. Local chemotherapy with slowly-releasing anticancer drug-polymers for malignant brain tumors. Journal of Controlled Release. 32:1–8 (1994).
K. A. Walter, M. A. Cahan, A. Gur, B. Tyler, J. Hilton, O. M. Colvin, P. C. Burger, A. Domb and H. Brem. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Research. 54:2207–2212 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winternitz, C.I., Jackson, J.K., Oktaba, A.M. et al. Development of a Polymeric Surgical Paste Formulation for Taxol. Pharm Res 13, 368–375 (1996). https://doi.org/10.1023/A:1016032207246
Issue Date:
DOI: https://doi.org/10.1023/A:1016032207246