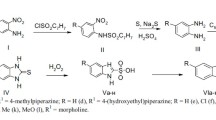
Abstract
The synthesis of five 4-aminouracil derivatives has been described. These compounds, which are chemically related to xanthines, were tested for possible diuretic activity. The increase in urine output by compounds I, III, and V was comparable to that produced by caffeine in an equimolar dose (5 × 10−3 M). On the isolated rabbit's heart (Langendorff s preparation), compounds I, III, and V (5 × 10−5 M) significantly increased the amplitude of contractions without having any significant effect on heart rate. Only these three compounds (4.4 x 10−8 M) relaxed the isolated rabbit thoracic aorta and rat anococcygeus smooth muscle which were precontracted with norepinephrine (4 × 10−8 M). This action was not antagonized by atropine, propranolol, or methylene blue, ruling out the involvement of acetylcholine, beta receptors, or endothelium-derived relaxing factor (EDRF). The relaxant effect, however, was reversed by the addition of calcium chloride, suggesting that this relaxation may be due to inhibition of the entry of extracellular calcium into the cells.
Similar content being viewed by others
REFERENCES
J. W. Daly. J. Med. Chem. 25:197–207 (1982).
J. D. Rutherford, S. F. Vanter, and E. Braunwald. Circulation 63:378–387 (1981).
T. W. Rall and T. C. West. J. Pharmacol. Exp. Ther. 139:269–274 (1963).
P. Hedqvist, B. B. Fredholm, and S. O'Lundh. Circ. Res. 43:592–598 (1978).
O. Schmiedeberg. Deutsches Ark. Klin. Med. 82:395–408 (1905).
H. Osswald. Naunyn-Schmiedeberg Arch. Pharmacol. 288:79–86 (1975).
J.-L. Bernier, J. P. Heinchart, and V. Warin. J. Heterocycl. Chem. 21:1129–1134 (1984).
J.-L. Bernier, J. P. Heinchart, and V. Warin. J. Med. Chem. 28:497–502 (1985).
G. Strauss. Liebigs Ann. 638:205–212 (1960).
W. Pfleiderer and K. H. Schundehutte. Liebigs Ann. 612:158–163 (1958).
R. F. Furchgott and S. Bhadrakom. J. Pharmacol. Exp. Ther. 108:129–143 (1953).
J. S. Gillespie. Br. J. Pharmacol. 45:404–416 (1972).
B. Sigurd and K. H. Olesen. Acta Med. Scand. 203:113–119 (1978).
D. F. Bohr. Science 139:597–599 (1963).
P. D. Turlapaty, R. K. Hester, and O. Carrier, Jr. Blood Vessels 13:193–209 (1976).
R. J. Heaslip and R. G. Rahwan. J. Pharmacol. Exp. Ther. 221:7–13 (1982).
R. E. Vestal, C. E. Ericksson, Jr., B. Musser, L. K. Ozaki, and J. B. Halter. Circulation 67:162–171 (1983).
R. Vinegar, J. F. Truax, J. L. Selph, R. M. Welch, and H. L. White. Proc. Soc. Exp. Biol. Med. 151:556–560 (1976).
M. G. Collis, G. S. Baxter, and J. R. Keddie. J. Pharm. Pharmacol. 38:850–852 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mustafa, A.A., Alhaider, A.A. & Hijazi, A.A. Study on the Renal and Cardiovascular Activities of Aminouracil Derivatives. Pharm Res 6, 394–398 (1989). https://doi.org/10.1023/A:1015979314978
Issue Date:
DOI: https://doi.org/10.1023/A:1015979314978