Abstract
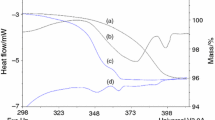
The hygroscopicity behavior of the pentahydrate, monohydrate, amorphous, and dehydrated forms of solid cefazolin sodium (CEZ) was studied under different relative humidity (RH) conditions. Between 42 and 86% RH, the pentahydrate (α form), the monohydrate, and their dehydrated forms absorbed atmospheric moisture equivalent to their hydrate numbers. The pentahydrate demonstrated a hysteresis effect at 15 and 31% RH. On the other hand, the water content of the amorphous form increased linearly with increases in RH. The noncrystalline state was maintained below 56% RH. For the dehydrated α form there was a distinct birefringence when viewed under polarizing light, the X-ray diffraction pattern was weak and diffuse, and the infrared (IR) spectra were discernibly different from that of the amorphous form. The freeze-dried CEZ showed hygroscopic behavior almost similar to that of the dehydrated α form. Two-component mixtures of various CEZ forms showed a linear relationship between the water content and the mixing ratio when stored at 31, 42, and 56% RH. From the hygroscopicity data, the crystallinity of freeze-dried CEZ could be estimated as the percentage of the dehydrated α form.
Similar content being viewed by others
REFERENCES
K. Kariyone, H. Harada, M. Kurita, and R. Tankano. J. Antibiot. 23:131–136 (1970).
M. Bornstein and S. M. Carone. U.S. Patent No. 4,002,748 (1977).
M. D. Cise and H. E. Osborne. U.S. Patent No. 4,104,470 (1978).
M. Bornstein and M. D. Cise. U.S. Patent No. 4,146,971 (1979).
L. Gatlin and P. P. DeLuca. J. Parent. Drug Assoc. 34:398–408 (1980).
Y. Koyama, M. Kamat, R. J. De Angelis, R. Srinivasan, and P. P. DeLuca. J. Parent. Sci. Technol. 42: 47–52 (1988).
M. D. Cise and M. L. Roy. U.S. Patent No. 4,132,848 (1979).
M. Inoue, K. Shima, and K. Imazu. Yakugaku Zasshi 104: 1268–1274 (1984).
M. Inoue, H. Tanaka, K. Shima, and K. Imazu. Yakugaku Zasshi 105:289–295 (1985).
N. A. Williams and G. P. Polli. J. Parent. Sci. Technol. 38:48–59 (1984).
M. J. Pikal, A. L. Lukes, J. E. Lang, and K. Gaines. J. Pharm. Sci. 67:767–773 (1978).
S. Y. Byrn, G. Gray, R. R. Pfeiffer, and J. Frye. J. Pharm. Sci. 74:565–568 (1985).
M. Bornstein, S. M. Carone, P. N. Thomas, and D. L. Coleman. Drug Dev. Indust. Pharm. 4:333–343 (1978).
G. S. Banker and C. T. Rhodes (eds.). Drug and the Pharmaceutical Sciences, Vol. 7, Marcel Dekker, New York, 1979.
A. Briggs. Dev. Biol. Stand. 36:251–260 (1977).
J. D. Gupta and R. B. Beevers. Chem. Rev. 62:665–668 (1962).
L. P. Marelli. Anal. Profiles Drug Subst. 4:21–47 (1975).
L. Lachman, H. A. Lieberman, and J. L. Kanig (eds.). The Theory and Practice of Industrial Pharmacy, Lea & Febiger, Philadelphia, 1970, pp. 32–34.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Osawa, T., Kamat, M.S. & DeLuca, P.P. Hygroscopicity of Cefazolin Sodium: Application to Evaluate the Crystallinity of Freeze-Dried Products. Pharm Res 5, 421–425 (1988). https://doi.org/10.1023/A:1015932316622
Issue Date:
DOI: https://doi.org/10.1023/A:1015932316622