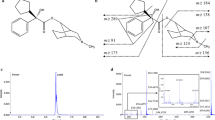
Abstract
The valepotriates, a group of chemically unstable iridoid triesters possessing sedative activity, contain various ester groups at the C-l, C-7, and C-11 positions. Using the selective INEPT NMR technique and employing a suitable polarization delay for long-range coupling, it was possible to achieve the assignment and location of the ester groups directly, without ambiguity, and without chemical modification. Six valepotriates isolated from Valmane tablets served as examples to demonstrate the utility of this NMR technique. During the course of this work, the “acevaltrate” fraction was shown to be a mixture of 1-α-ace valtrate (3) and 7-β-acevaltrate (4), the structures of valtrate (1) and didrovaltrate (2) were confirmed directly, and two new valepotriates, 5a and 5b, were obtained as an inseparable mixture and characterized.
Similar content being viewed by others
REFERENCES
M. Grieve. A Modern Herbal, Vol. II, Hafner, New York, 1959 (reprinted; originally published in 1931), pp. 824–830.
C. Hobbs. Valerian (Valeriana officinalis)—a literature review. HerbalGram No. 21:19–34 (1989).
P. J. Houghton. The biological activity of valerian and related plants. J. Ethnopharmacol. 22:121–142 (1988).
G. E. Trease and W. C. Evans. Pharmacognosy, 11th ed., Baillière Tindall, London, 1978, pp. 460–462.
V. E. Tyler. Plight of plant-drug research in the United States today. Econ. Bot. 33:377–383 (1979).
V. E. Tyler. The Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies, George F. Stickley, Philadelphia, 1982, pp. 17–18, 224–225.
V. E. Tyler, L. R. Brady, and J. E. Robbers. Pharmacognosy, 9th ed., Lea & Febiger, Philadelphia, 1988, p. 491.
Rote Liste 1990, Hypnotika/sedativa. Bundesverband der Pharmazeutischen Industrie e.V., Editio Cantor (Aulendorff/Württ), Berlin/Munich, group 48.
J. E. F. Reynolds (ed.). Martindale: The Extra Pharmacopoeia, 29th ed., Pharmaceutical Press, London, 1989, p. 1628.
O. Sticher. Plant mono, di-and sesquiterpenoids with pharmacological and therapeutical activity. In H. Wagner and P. Wolff (eds.), New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity, Springer, New York, 1977, pp. 137–176.
P. W. Thies, E. Finner, and S. David. On the active agents of valerian. XIV. Assignment of type and location of the acyloxy substituents in valepotriates via C13-NMR spectroscopy. Planta Med. 41:15–20 (1981).
S. S. Popov and N. V. Handjieva. Mass spectrometry of valepotriates. Biomed. Mass Spectrom. 6:124–128 (1979).
P. W. Thies. Die Konstitution der Valepotriate. Mitteilung über die Wirkstoffe des Baldrians. Tetrahedron 24:313–347 (1968).
A. Bax. Structure determination and spectral assignment by pulsed polarization transfer via long-range 1H-13C coupling. J. Magn. Reson. 57:314–318 (1984).
G. A. Cordell. NMR techniques for the structure elucidation and conformational analysis of natural products. Kor. J. Pharmacog. 19:153–169 (1988).
G. A. Cordell. Selective INEPT spectroscopy—a powerful tool for the spectral assignment and structure elucidation of natural products. Phytochem. Anal. 2:49–59 (1991).
H. Wagner, S. Bladt, and E. M. Zgainski. Plant Drug Analysis:A Thin-Layer Chromatography Atlas, Springer, New York, 1984, pp. 263–267.
P. W. Thies. Über die Wirkstoffe des Baldrians 2: Zur Konstitution der Isovaleriansäurester Valepotriat, Acetoxyvalepotriat und Dihydrovalepotriat. Tetrahedron Lett. 11:1163–1170 (1966).
W. Foerster, H. Becker, and E. Rodriguez. HPLC analysis of valepotriates in the North American genera Plectritis and Valeriana. Planta Med. 50:7–9 (1984).
H. Wagner and K. Jurcic. On the spasmolytic activity of Valeriana extracts. Planta Med. 37:84–86 (1979).
C. Bounthanh, C. Bergmann, J. P. Beck, M. Haag-Berrurier, and R. Anton. Valepotriates, a new class of cytotoxic and antitumor agents. Planta Med. 41:21–28 (1981).
W. von der Hude, M. Scheutwinkel-Reich, R. Braun, and W. Dittmar. In vitro mutagenicity of valepotriates. Arch. Toxicol. 56:267–271 (1985).
M. Tortarolo, R. Braun, G. E. Hubner, and H. R. Maurer. In vitro effects of epoxide-bearing valepotriates on mouse early hematopoietic progenitor cells and human T-lymphocytes. Arch. Toxicol. 51:37–42 (1982).
C. Bounthanh, L. Richert, J. P. Beck, M. Haag-Berrurier, and R. Anton. The action of valepotriates on the synthesis of DNA and proteins of cultured hepatoma cells. Planta Med. 49:138–142 (1983).
G. Tittel, V. M. Chari, and H. Wagner. HPLC-Analysis of Valeriana mexicana extracts. Planta Med. 34:305–310 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, LJ., Cordell, G.A. & Balandrin, M.F. Valerian-Derived Sedative Agents. I. On the Structure and Spectral Assignment of the Constituents of Valmane Using the Selective INEPT Nuclear Magnetic Resonance Technique. Pharm Res 8, 1094–1102 (1991). https://doi.org/10.1023/A:1015889915326
Issue Date:
DOI: https://doi.org/10.1023/A:1015889915326