Abstract
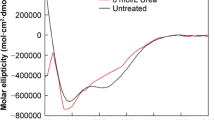
Bile salts have been found to be effective absorption promoters of insulin across mucosal barriers, i.e., nasal and gastrointestinal. One of the mechanisms proposed for absorption enhancement is the dissociation of insulin oligomers to monomers, rendering a higher insulin diffusivity. α-Chymotryptic degradation and circular dichroism studies were used to characterize such a transition. When zinc insulin (hexamers) and sodium insulin (dimers) were subjected to α-chymotryptic degradation, a 3.2-fold difference in the apparent first-order rate constants was observed (zinc insulin being slower than sodium insulin), representing the intrinsic difference in the concentration of total associated species in solution (three times). In the presence of a bile salt, sodium glycocholate (NaGC), the rate of degradation of both zinc and sodium insulin increased in an asymptotic manner. A maximum increase of 5.4-fold was observed for zinc insulin at a 30 mM NaGC concentration and a 2.1-fold increase was noted for sodium insulin at 10 mM NaGC, both values being close to the theoretical numbers of 6- and 2-fold as predicted by the complete dissociation of hexamers and dimers to monomers. The result indicates dissociation of insulin oligomers to monomers by bile salt micelles, probably by hydrophobic micellar incorporation of monomeric units. Circular dichroism studies also revealed progressive attenuation of molecular ellipticities at negative maxima of 276, 222, and 212 nm for zinc insulin solution in the presence of NaGC. Therefore, both α-chymotryptic degradation and circular dichroism studies have consistently demonstrated that the bile salts may be capable of dissociating insulin oligomers to monomers, a fact which may play an important role in enhancing insulin bioavailability.
Similar content being viewed by others
REFERENCES
R. Quinn and J. D. Andrade. Minimizing the aggregation of neutral insulin solution. J. Pharm. Sci. 72:1472–1483 (1983).
S. Sato, C. D. Ebert, and S. W. Kim. Prevention of insulin self-association and surface adsorption. J. Pharm. Sci. 72:228–232 (1983).
A. M. Alibisser, W. Longheed, K. Perlman, and A. Bahoric. Nonaggregating insulin solutions for long-term glucose control in experimental and human diabetes. Diabetes 29:241–243 (1980).
E. Mosekilde, K. S. Jensen, C. Binder, S. Pramming, and B. Thorsteinsson. Modeling absorption kinetics of subcutaneous injected soluble insulin. J. Pharmacokin. Biopharm. 17:67–87 (1989).
P. Tengamnuay and A. K. Mitra. Bile salt-fatty acid mixed micelles as nasal absorption promoters of peptides. II. In vivo nasal absorption of insulin in rats and effect of mixed micelles on the morphological integrity of the nasal mucosa. Pharm. Res. 7:370–375 (1990).
S. Hirai, T. Yashiki, and H. Mima. Mechanisms for the enhancement of nasal absorption of insulin by surfactants. Int. J. Pharm. 9:173–184 (1981).
M. D. Donovan, G. L. Flynn, and G. L. Amidon. The molecular weight dependence of nasal absorption: The effect of absorption enhancers. Pharm. Res. 7:808–815 (1990).
G. S. Gordon, A. C. Moses, R. D. Silvers, J. S. Flier, and M. C. Carey. Nasal absorption of insulin: Enhancement by hydrophobic bile-salts. Proc. Natl. Acad. Sci. USA 82:7419–7423 (1985).
R. J. Schilling and A. K. Mitra. Intestinal mucosal transport of insulin. Int. J. Pharm. 62:53–64 (1990).
R. J. Schilling and A. K. Mitra. Degradation of insulin by trypsin and α-chymotrypsin. Pharm. Res. 8:721–727 (1991).
F-y. Liu, D. O. Kildsig, and A. K. Mitra. Insulin aggregation in aqueous media and its effect on alpha-chymotrypsin-mediated proteolytic degradation. Pharm. Res. 8:925–929 (1991).
A. Ginsburg and H. K. Schachman. Studies on the enzymatic breakdown of proteins. I. Action of chymotrypsin on insulin. J. Biol. Chem. 235:108–114 (1960).
P. Tengamnuay. Nasal Absorption of Selected Amino Acids and Peptides in Rats, Ph.D. thesis, Purdue University, West Lafayette, IN, 1989.
J. Goldman and F. H. Carpenter. Zinc binding, circular dichroism, and equilibrium sedimentation studies on insulin (bovine) and several of its derivatives. Biochemistry 13:4566–4574 (1974).
Z. Shao and A. K. Mitra. Membrane protein and enzyme release by bile salts and bile salt-fatty acid mixed micelles—Correlation with facilitated nasal drug transport. Pharm. Res. (in press).
F. G. J. Poelma, R. Breas, and J. J. Tukker. Intestinal absorption of drugs. IV. The influence of taurocholate and L-cysteine on the barrier function of mucus. Int. J. Pharm. 64:161–169 (1990).
E. L. Smith, R. L. Hill, and A. Borman. Activity of insulin degraded by leucine aminopeptidase. J. Biol. Chem. 29:207–208 (1958).
D. Crowfoot, D. C. Hodgkin, E. Dodson, G. Dodson, and C. Reynolds. Structure and function of proteins and nucleic acids. Biochem. Soc. Trans. 11:411–417 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Y., Shao, Z. & Mitra, A.K. Dissociation of Insulin Oligomers by Bile Salt Micelles and Its Effect on α-Chymotrypsin-Mediated Proteolytic Degradation. Pharm Res 9, 864–869 (1992). https://doi.org/10.1023/A:1015888529728
Issue Date:
DOI: https://doi.org/10.1023/A:1015888529728