Abstract
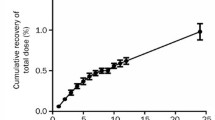
The pharmacokinetic parameters of leuprolide acetate, a potent analogue of LH-RH, were determined in rats and dogs after i.v. and s.c. dosing with leuprolide solution. The effective human dose of once-a-month injectable microspheres of leuprolide was estimated to be about 3.2 to 8.1 mg analogue/month using these parameters. After microsphere injection at three different doses in rat serum leuprolide concentrations were sustained for over 4 weeks, and the AUCs and mean serum levels were linearly correlated with the dose. The serum levels and urinary excretion of the analogue in rats after repeated s.c. injection of the microspheres every 4 weeks exhibited similar profiles after each injection; no changes of the absorption and excretion of the analogue after the repeated injection could be demonstrated. The serum levels of the analogue metabolite (M-I) were 21% of the intact form 3 hr after injection of the microspheres but very low at the steady state after 1 to 4 weeks.
Similar content being viewed by others
REFERENCES
H. Okada. One-month release injectable microspheres of leuprolide acetate, a superactive agonist of LH-RH. Proc. Int. Symp. Control. Rel. Bioact. Mater. 16:12–13 (1989).
H. Okada, Y. Ogawa, and T. Yashiki. Prolonged release microcapsules and its production. U.S. Patent 4652441 (1987).
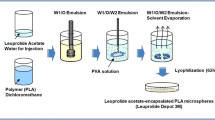
Y. Ogawa, M. Yamamoto, H. Okada, T. Yashiki, and T. Shimamoto. A new technique to efficiently entrap leuprolide acetate into microcapsules of polylactic acid or copoly-(lactic/glycolic) acid. Chem. Pharm. Bull. 36:1095–1103 (1988).
M. B. Garnick and the leuprolide study group. Leuprolide versus diethylstilbestrol for metastatic cancer. N. Engl. Med. 311:1281–1286 (1984).
H. Okada, Y. Sakura, T. Kawaji, T. Yashiki, and H. Mima. Regression of rat mammary tumors by a potent luteinizing hormone-releasing hormone analogue (leuprolide) administered vaginally. Cancer Res. 43:1869–1874 (1983).
H. Okada, T. Heya, Y. Ogawa, and T. Shimamoto. One-month release injectable microspheres of a luteinizing hormone-releasing hormone agonist (leuprolide acetate) for treating experimental endometriosis in rats. J. Pharmacol. Exp. Ther. 244:744–750 (1988).
H. Okada, T. Heya, Y. Igari, Y. Ogawa, H. Toguchi, and T. Shimamoto. One-month release injectable microspheres of leuprolide acetate inhibit steroidogenesis and genital organ growth in rats. Int. J. Pharm. 54:231–239 (1989).
Y. Ogawa, H. Okada, T. Heya, and T. Shimamoto. Controlled release of LHRH agonist, leuprolide acetate, from microcapsules: Serum drug level profiles and pharmacological effects in animals. J. Pharm. Pharmacol. 41:439–444 (1989).
H. Okada, T. Heya, Y. Ogawa, H. Toguchi, and T. Shimamoto. Sustained pharmacological activities in rats following single and repeated administration of once-a-month injectable microspheres of leuprolide acetate. Pharm. Res. 8:584–587 (1991).
H. Ueno and S. Matsuo. High-performance liquid chromatography followed by radioimmunoassay for the determination of a luteinizing hormone-releasing hormone analogue, leuprorelin and its metabolite. J. Chromatogr. (in press).
H. Okada, I. Yamazaki, T. Yashiki, T. Shimamoto, and H. Mima. Vaginal absorption of a potent luteinizing hormone-releasing hormone analog (leuprolide) in rats. IV. Evaluation of the vaginal absorption and gonadotropin responses by radioimmunoassay. J. Pharm. Sci. 73:298–302 (1984).
L. T. Sennello, R. A. Finley, S.-Y. Chu, C. Jagst, D. Max, D. E. Rollins, and K. G. Tolman. Single-dose pharmacokinetics of leuprolide in humans following intravenous and subcutaneous administration. J. Pharm. Sci. 75:158–160 (1986).
R. Sharifi, M. Soloway, and the Leuprolide Study Group. Clinical study of leuprolide depot formulation in the treatment of advanced prostate cancer. J. Urol. 143:68–71 (1990).
I. Naeshiro, T. Kondo, M. Mitani, K. Yoshida, T. Kobayashi, T. Kimura, H. Shimamura, and S. Tanayama. Metabolic fate of TAP-144, an LH-RH agonist, in rats and dogs. Jpn. Pharmacol. Ther. 18:35–58 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okada, H., Inoue, Y., Heya, T. et al. Pharmacokinetics of Once-a-Month Injectable Microspheres of Leuprolide Acetate. Pharm Res 8, 787–791 (1991). https://doi.org/10.1023/A:1015818504906
Issue Date:
DOI: https://doi.org/10.1023/A:1015818504906