Abstract
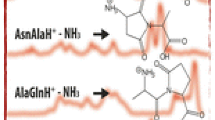
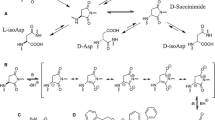
Deamidation of Asn residues is a major chemical pathway of degradation of peptides and proteins. To understand better the external factors that influence deamidation, we studied the degradation of the hexapeptide Val–Tyr–Pro–Asn–Gly–Ala, a fragment of adrenocorticotropic hormone, by HPLC. The deamidation of this model peptide showed marked dependence on pH, temperature, and buffer composition. In the pH range 5 to 12, the peptide deamidated exclusively via a cyclic imide intermediate with the formation of both the Asp- and the isoAsp-hexapeptides. Buffer catalysis was also observed in the pH range of 7 to 11. However, at acidic pH's, the pathway of deamidation involved direct hydrolysis of the amide side chain of Asn residue to produce only the Asp-hexapeptide.
Similar content being viewed by others
REFERENCES
A. B. Robinson and C. J. Rudd. In B. L. Horecker and E. R. Stadtman (eds.), Current Topics in Cellular Regulations, Vol. 8, Academic Press, New York, 1974, pp. 247–295.
P. Bornstein and G. Balian. Methods Enzymol. 47:132–145 (1977).
T. Geiger and S. Clarke. J. Biol. Chem. 262:785–794 (1987).
R. Lura and V. Schirch. Biochemistry 27:7671–7677 (1988).
Y. C. Meinwald, E. R. Stimson, and H. A. Scheraga. Int. J. Peptide Protein Res. 28:79–84 (1986).
M. A. Ondetti, A. Deer, J. T. Sheehan, J. Pluscec, and O. Kocy. Biochemistry 7:4069–4075 (1968).
S. Capasso, L. Mazzarella, F. Sica, and A. Zagari. Pept. Res. 2:195–200 (1989).
P. J. M. van den Oestelaar and H. J. Hoenders. Adv. Exp. Med. Biol. 231:261–267 (1988).
R. C. Stephenson and S. Clarke. J. Biol. Chem. 264:6164–6170 (1989).
M. Bodanszky and J. Martinez. Synthesis 333–356 (1981).
T. Flatmark. Acta Chem. Scand. 20:1487–1496 (1966).
K. U. Yuksel and R. W. Gracy. Arch. Biochem. Biophys. 248:452–459 (1986).
D. W. Aswad. J. Biol Chem. 259:10714–10721 (1984).
B. A. Johnson and D. W. Aswad. Biochemistry 24:2581–2586 (1985).
N. P. Bhatt, K. Patel, and R. T. Borchardt. Pharm. Res. 7:593–599 (1990).
E. D. Murray, Jr., and S. Clarke. J. Biol. Chem. 259:10722–10732 (1984).
J. P. Tam, M. W. Riemer, and R. B. Merrifield. Pept. Res. 1:6–18 (1988).
I. Schon and L. Kisfaludy. Int. J. Peptide Protein Res. 14:485–494 (1979).
J. P. Tam, T. W. Wong, M. W. Rieman, F. S. Tjoeng, and R. B. Merrifield. Tetrahedron Lett. 42:4033–4036 (1979).
C. C. Yang and R. B. Merrifield. J. Org. Chem. 41:1032–1041 (1976).
T. Baba, H. Sugiyama, and S. Seto. Chem. Pharm. Bull. 21:207–209 (1973).
J. Stewart and J. S. Young. In Solid Phase Peptide Synthesis, 2nd ed., Pierce, Rockford, IL, 1984, pp. 71–95.
G. Allen. Sequencing of Proteins and Peptides, North-Holland, Amsterdam, 1981.
I. H. Segel. Biochemical Calculations, John Wiley and Sons, New York, 1968.
G. R. Drapeau. Methods Enzymol. 45:469–475 (1976).
A. S. Inglis. Methods Enzymol. 91:324–332 (1983).
T. J. Ahern and A. M. Klibanov. Science 228:1280–1284 (1985).
S. E. Zale and A. M. Klibanov. Biochemistry 25:5432–5444 (1986).
I. M. Ota, L. Ding, and S. Clarke. J. Biol. Chem. 262:8522–8531 (1987).
I. M. Ota and S. Clarke. Biochemistry 28:4020–4027 (1989).
L. E. Kirsch, R. M. Molloy, M. Debono, P. Baker, and K. Z. Farid. Pharm. Res. 6:387–393 (1989).
A. Di Donato, P. Galletti, and G. D'Alessio. Biochemistry 25:8361–8368 (1986).
F. Araki, H. Nakamura, N. Nojima, K. Tsukumo, and S. Sakamoto. Chem. Pharm. Bull. 37:404–406 (1989).
S. Clarke. Int. J. Peptide Protein Res. 30:808–821 (1987).
C. K. Sauers, C. A. Marikakis, and M. A. Lupton. J. Am. Chem. Soc. 95:6792–6799 (1973).
M. L. Ernst and G. L. Schmir. J. Am. Chem. Soc. 88:5001–5009 (1966).
S. A. Bemhard, A. Berger, J. H. Carter, E. Katchalski, M. Sela, and Y. Shelitiny. J. Am. Chem. Soc. 84:2421–2434 (1962).
B. A. Johnson, E. D. Murray, Jr., S. Clarke, D. B. Glass, and D. W. Aswad. J. Biol. Chem. 262:5622–5629 (1987).
M. Bodanszky and J. Z. Kwei. Int. J. Peptide Protein Res. 12:69–74 (1978).
P. N. McFadden and S. Clarke. Proc. Natl. Acad. Sci. USA 84:2595–2599 (1987).
E. D. Murray, Jr., and S. Clarke. J. Biol. Chem. 261:306–313 (1986).
P. N. McFadden and S. Clarke. J. Biol. Chem. 261:11503–11511 (1986).
E. Tanford. Adv. Protein Chem. 17:69–165 (1962).
A. Fersht. In Enzyme Structure and Mechanism, 2nd ed., W. H. Freeman, New York, 1985, pp. 155–175.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Patel, K., Borchardt, R.T. Chemical Pathways of Peptide Degradation. II. Kinetics of Deamidation of an Asparaginyl Residue in a Model Hexapeptide. Pharm Res 7, 703–711 (1990). https://doi.org/10.1023/A:1015807303766
Issue Date:
DOI: https://doi.org/10.1023/A:1015807303766