Abstract
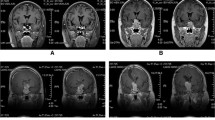
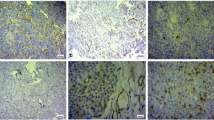
Although pituitary adenomas often recur, a reliable predictor for their recurrences has not yet been established. The aim of this study is to assess the utility of the nuclear accumulation of basic fibroblast growth factor (bFGF) as a predictor for the recurrence of pituitary adenomas. We studied 64 patients who had primary pituitary adenomas and underwent operations. The immunohistochemistry for bFGF and MIB-1 was retrospectively examined in paraffin-embedded tissues. The bFGF immunoreactivity in the cytoplasm was assigned one of four grades and the bFGF immunoreactivity in the nucleus was recorded as the bFGF nuclear index (NI), which was calculated as a percentage of tumor cells with the bFGF immunoreactivity in the nuclei in more than 1000 tumor cells. Recurrent adenomas were found in 7 patients during follow-up periods ranging from 8 to 134 months (mean: 57.3). Kaplan–Meier analysis demonstrated that high bFGF NI (>30%) correlated with poor recurrence free rate (p<0.02). We assessed the relative contribution of bFGF NI to recurrence free by using multivariate (Cox's proportional hazards model) analyses with variable factors. Multivariate analysis showed that only bFGF NI was a potential predictor of recurrence free, independent of all other variables. High bFGF NI (>30%) had a relative risk of 8.9, with a 95% confidence interval of 1.0–74.9 (p<0.05). We suggest that the bFGF NI may be a potentially useful predictor for the recurrence of pituitary adenomas.
Similar content being viewed by others
References
Bochicchio D, Losa M, Buchfelder M: Factors influencing the immediate and late outcome of Cushing's disease treated by transsphenoidal surgery: a retrospective study by the European Cushing's Disease Survey Group. J Clin Endocrinol Metab 80: 3114-3120, 1995
Ebersold MJ, Quast LM, Laws ER Jr, Scheithauer B, Randall RV: Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J Neurosurg 64: 713-719, 1986
Feigenbaum SL, Downey DE, Wilson CB, Jaffe RB: Transsphenoidal pituitary resection for preoperative diagnosis of prolactin-secreting pituitary adenoma in women: long term follow-up. J Clin Endocrinol Metab 81: 1711-1719, 1996
Ho DM, Hsu CY, Ting LT, Chiang H: The clinicopathological characteristics of gonadotroph cell adenoma: a study of 118 cases. Hum Pathol 28: 905-911, 1997
Knappe UJ, Ludecke DK: Persistent and recurrent hypercortisolism after transsphenoidal surgery for Cushing's disease. Acta Neurochir Suppl (Wien) 65: 31-34, 1996
Laws ER, Thapar K: Recurrent pituitary adenomas. In: Landolt AM, Vance ML, Reilly PL (eds) Pituitary Adenomas. Churchill Livingstone, New York, 1996, pp 385-393
Rauhut F, Clar HE, Bamberg M, Benker G, Grote W: Diagnostic criteria in pituitary tumour recurrence-combined modality of surgery and radiotherapy. Acta Neurochir (Wien) 80: 73-78, 1986
Atkin SL, Green VL, Hipkin LJ, Landolt AM, Foy PM, Jeffreys RV, White MC: A comparison of proliferation indices in human anterior pituitary adenomas using formalin-fixed tissue and in vitro cell culture. J Neurosurg 87: 85-88, 1997
Abe T, Sanno N, Osamura YR, Matsumoto K: Proliferative potential in pituitary adenomas: measurement by monoclonal antibody MIB-1. Acta Neurochir (Wien) 139: 613-618, 1997
Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Maira G: Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol 52: 107-111, 1999
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER Jr: Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38: 99-106, 1996
Zhao D, Tomono Y, Nose T: Expression of P27kip1 and Ki-67 in pituitary adenomas: an investigation of marker of adenoma invasiveness. Acta Neurochir (Wien) 141: 187-192, 1999
Hsu DW, Hakim F, Biller BM, de la Monte S, Zervas NT, Klibanski A, Hedley-Whyte ET: Significance of proliferating cell nuclear antigen index in predicting pituitary adenoma recurrence. J Neurosurg 78: 753-761, 1993
Bouche G, Gas N, Prats H, Baldin V, Tauber JP, Teissie J, Amalric F: Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0-G1 transition. Proc Natl Acad Sci USA 84: 6770-6774, 1987
Joy A, Moffett J, Neary K, Mordechai E, Stachowiak EK, Coons S, Rankin-Shapiro J, Florkiewicz RZ, Stachowiak MK: Nuclear accumulation of FGF-2 is associated with proliferation of human astrocytes and glioma cells. Oncogene 14: 171-183, 1997
Miyaji K, Tani E, Nakano A, Ikemoto H, Kaba K: Inhibition by 5'-methylthioadenosine of cell growth and tyrosine kinase activity stimulated by fibroblast growth factor receptor in human gliomas. J Neurosurg 83: 690-697, 1995
Suzui H, Takahashi JA, Fukumoto M, Hashimoto N, Itoh N, Hatanaka M, Kikuchi H: Immunohistochemical study for basic fibroblast growth factor and fibroblast growth factor receptor I in pituitary adenomas. Neurosci Lett 171: 192-196, 1994
Arese M, Chen Y, Florkiewicz RZ, Gualandris A, Shen B, Rifkin DB: Nuclear activities of basic fibroblast growth factor: potentiation of low-serum growth mediated by natural or chimeric nuclear localization signals. Mol Biol Cell 10: 1429-1444, 1999
Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P: Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol 12: 240-247, 1992
Bailly K, Soulet F, Leroy D, Amalric F, Bouche G: Uncoupling of cell proliferation and differentiation activities of basic fibroblast growth factor. FASEB J 14: 333-344, 2000
Maher P: p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J Biol Chem 274: 17491-17498, 1999
Wang X, Weng LP, Yu Q: Specific inhibition of FGF-induced MAPKactivation by the receptor-like protein tyrosine phosphatase LAR. Oncogene 19: 2346-2353, 2000
Bikfalvi A, Klein S, Pintucci G, Quarto N, Mignatti P, Rifkin DB: Differential modulation of cell phenotype by different molecular weight forms of basic fibroblast growth factor: possible intracellular signaling by the high molecular weight forms. J Cell Biol 129: 233-243, 1995
Pintucci G, Quarto N, Rifkin DB: Methylation of high molecular weight fibroblast growth factor-2 determines post-translational increases in molecular weight and affects its intracellular distribution. Mol Biol Cell 7: 1249-1258, 1996
Knosp E, Steiner E, Kitz K, Matula C: Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33: 610-617, 1993
Hu G, Kim H, Xu C, Riordan JF: Fibroblast growth factors are translocated to the nucleus of human endothelial cells in a microtubule-and lysosome-independent pathway. Biochem Biophys Res Commun 273: 551-556, 2000
Stachowiak EK, Maher PA, Tucholski J, Mordechai E, Joy A, Moffett J, Coons S, Stachowiak MK: Nuclear accumulation of fibroblast growth factor receptors in human glial cells-association with cell proliferation. Oncogene 14: 2201-2211, 1997
Stachowiak MK, Moffett J, Maher P, Tucholski J, Stachowiak EK: Growth factor regulation of cell growth and proliferation in the nervous system.Anewintracrine nuclear mechanism. Mol Neurobiol 15: 257-283, 1997
Maher PA: Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J Cell Biol 134: 529-536, 1996
Beyer AL, Christensen ME, Walker BW, LeStourgeon WM: Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell 11: 127-138, 1977
Lischwe MA, Cook RG, Ahn YS, Yeoman LC, Busch H: Clustering of glycine and NG,NG-dimethylarginine in nucleolar protein C23. Biochemistry 24: 6025-6028, 1985
Lischwe MA, Ochs RL, Reddy R, Cook RG, Yeoman LC, Tan EM, Reichlin M, Busch H: Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem 260: 14304-14310, 1985
Delehedde M, Boilly B, Hondermarck H: Differential responsiveness of human breast cancer cells to basic fibroblast growth factor: a cell kinetics study. Oncol Res 7: 399-405, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fukui, S., Otani, N., Nawashiro, H. et al. Nuclear Accumulation of Basic Fibroblast Growth Factor as a Predictor for the Recurrence of Pituitary Adenomas. J Neurooncol 57, 221–229 (2002). https://doi.org/10.1023/A:1015763725104
Issue Date:
DOI: https://doi.org/10.1023/A:1015763725104