Abstract
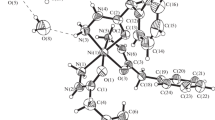
[Mn(H2O)3(phen)(C4H4O4)]·2H2O was obtained by reaction of freshly prepared MnCO3, phen and succinic acid in CH3OH/H2O (1:1 v/v), and its crystal structure has been determined by single crystal X-ray diffraction methods. The title mixed ligand complex crystallizes in the triclinic space group \(P\bar 1\) with cell dimensions a = 7.590(1) Å, b = 9.324(1) Å, c = 13.917(1) Å, α = 85.64(1)°, β = 74.56(1)°, γ = 77.10(1)°, and D calc = 1.584 g/cm3 for Z = 2. The crystal structure consists of the [Mn(H2O)3(phen)(C4H4O4)] complex molecules and lattice H2O molecules. The Mn atoms are each octahedrally coordinated by two N atoms of one phen ligand and four O atoms of three H2O molecules and one succinato ligand with d(Mn—N) = 2.271 and 2.299 Å, and d(Mn—O) = 2.133–2.239 Å. Through intermolecular hydrogen bondings, the complex molecules are interlinked to form 2D layers, which are assembled by π–π stacking interactions into 3D framework with tunnels occupied by the lattice H2O molecules. Thermal analyses showed that the title compound decomposes in two steps over the range 25–600°C upon heating in flowing Ar.
Similar content being viewed by others
References
Leiningger, S.; Olenyuk, B.; Stang, P.J. Chem. Rev. 2000, 100, 853.
Swiegers, G.F.; Malefetse, T.J. Chem. Rev. 2000, 100, 3483.
Fujita, M. Acc. Chem. Res. 1999, 32, 52.
Raymo, F.M.; Stoddart, J.F. Chem. Rev. 1999, 99, 1643.
Hagrman, P.J.; Zubieta, J. Inorg. Chem. 2001, 40, 2800.
Finn, R.C.; Zubieta, J. Inorg. Chem. 2001, 40, 2466.
Yu, S.-B.; Lippard, S.J.; Shweky, I.; Bino, A. Inorg. Chem. 1992, 31, 3502.
Vincent, J.B.; Tsai, H.-L.; Blackman, A.G.; Wang, S.; Boyd, P.D.W.; Folting, K.; Huffman, J.C.; Lobkovsky, E.B.; Hendrickson, D.N.; Christou, G. J. Am. Chem. Soc. 1993, 115, 1253.
Dimitrou, K.; Folting, K.; Streib, W.E.; Christou, G. J. Am. Chem. Soc. 1993, 115, 6432.
Corbella, M.; Costa, R.; Ribas, J.; Fries, P.H.; Latour, J.M.; Öhrström, L.; Solans, X.; Rodriguez, V. Inorg. Chem. 1996, 35, 1857.
Sugimori, T.; Masuda, H.; Ohata, N.; Koiwai, K.; Odani, A.; Yamauchi, O. Inorg. Chem. 1997, 36, 576.
Yamauchi, O.; Odani, A.; Masuda, A.; Sigel, H. Met. Ions Biol. Syst. 1996, 32, 207.
Dubler, E.; Haering, U.K.; Scheller, K.H.; Balter, P.; Sigel, H. Inorg. Chem. 1984, 20, 3785.
Munakata, M.; Wu, L.P.; Yamamoto, M.; Kuroda-Sowa, T.; Maekawa, M. J. Am. Chem. Soc. 1996, 118, 3117.
Dai, J.; Yamamoto, M.; Kuroda-Sowa, T.; Maekawa, M.; Suenaga, Y.; Munakata, M. Inorg. Chem. 1997, 36, 2688.
Mizutani, M.; Kubo, I.; Jitsukawa, K.; Masuda, H.; Einaga, H. Inorg. Chem. 1999, 38, 420.
Aumuller, A.; Erk, P.; Klebe, G.; Hunig, S.; Von Schutz, J.U.; Werner, H.-P. Angew. Chem., Int. Ed. Engl. 1986, 25, 740.
Ermer, O. Adv. Mater. 1991, 3, 608.
Robson, R.; Abrahams, B.F.; Batten, S.R.; Gable, R.W.; Hoskins, B.F.; Liu, J. Supramolecular Architetcture; ACS Symposium Series 449; American Chemical Society: Washington, DC, 1992; chap. 19.
Subramanian, S.; Zaworotko, M.J. Angew. Chem., Int. Ed. Engl. 1995, 34, 2127.
Teichert, O.; Sheldrick, W.S. Z. Anorg. Allg. Chem. 2000, 626, 1509.
Rao, C.N.R.; Ranganathan, A.; Pedireddi, V.R.; Raju, A.R. Chem. Commun. 2000, 39.
Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. J. Am. Chem. Soc. 1994, 116, 1151.
Wang, Z.; Xiong, R.G.; Foxman, B.M.; Wilson, S.R.; Lin, W. Inorg. Chem. 1999, 38, 1523, and references therein.
Gupta, M.P.; Sahu, R.D.; Ram, R.; Maulik, P.R. Z. Kristallogr. 1983, 163, 155.
Fleck, M.; Tillmanns, E.; Bohaty, L. Z. Kristallogr. NCS 2000, 215, 429.
Zheng, Y.Q.; Lin, J.L. Z. Kristallogr. NCS 2000, 215, 159.
Zheng, Y.Q.; Lin, J.L. Z. Kristallogr. NCS 2000, 215, 157.
Zheng, Y.Q.; Lin,J.L. Z. Kristallogr. NCS 2000, 215, 165.
O'Connor, B.H.; Maslen, E.N. Acta Crystallogr. 1966, 20, 824.
Griffith, E.A.H.; Charles, N.G.; Amma, E.L. Acta Crystallogr. Sect. B 1982, 38,262.
ichaelides, A.; Kiritsis, V.; Skoulika, S.; Aubry, A. Angew. Chem. 1993, 105, 1525. Mn(II) phen succinato complex 125
heng, Y.Q.; Peters, K.; von Schnering, H.G. Chem. Res. Chin. Univ. 2001, 17, 20.
Lin, J.L.; Zheng, Y.Q. J. Ningbo Univ. 2000, 13, 31.
Zheng, Y.Q.; Sun, J.; Lin, J.L. Z. Anorg. Allg. Chem. 2000, 626, 1501.
Zheng, Y.Q.; Sun, J.; Lin, J.L. Acta Chem. Sin. 2000, 58, 1131.
Zheng, Y.Q.; Sun, J.; Lin, J.L. Z. Anorg. Allg. Chem. 2001, 627, 1990.
Sheldrick, G.M. SHELXS-97, Programm zur Lösung von Kristallstrukturen; University of Göttingen: Germany, 1997.
Sheldrick, G.M. SHELXL-97, Programm zur Verfeinerung von Kristallstrukturen; University of Göttingen: Germany, 1997.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, YQ., Kong, ZP. Synthesis, crystal structure, and thermal analyses of triaqua-(1,10-phenanthroline-N,N′) succinatomanganese(II) dihydrate, [Mn(H2O)3(phen)(C4H4O4)]·2H2O. Journal of Chemical Crystallography 32, 119–125 (2002). https://doi.org/10.1023/A:1015697701786
Issue Date:
DOI: https://doi.org/10.1023/A:1015697701786