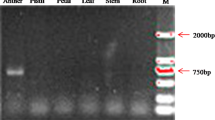
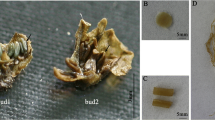
Abstract
Isopentenyl transferase (ipt) gene from Agrobacterium tumefaciens T-DNA was placed under the control of a TA29 promoter which expresses specifically in anther. The chimeric TA29-ipt gene was transferred to tobacco plants. During flowering, mRNA of the ipt gene in the anthers of the transgenic plants accumulated and the level of iPA + iPs increased 3–4-fold in the leaves, petals, pistils, and stamens compared with those in the wild type plants. This cytokinin increase affected various aspects in development indicating that the alterations of endogenous cytokinin level by using anther-specific expression of the TA29-ipt gene affected morphology, floral organ systems and reproductivity of the transgenic plants.
Similar content being viewed by others
References
Akiyoshi DE, Morris RO, Mischke BS, Kosuge T, Garfinkel D, Gordon MP and Nester EW (1983) Cytokinin-auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci USA 80: 407–411.
Akiyoshi D, Klee H, Amasino R, Nester EW and Gordon MP (1984) T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA 81: 5994–5998.
Beinsberger SEI, Valcke RLM, Clijsters HMM, De Greef JA and Van onkelen HA (1992) Effects of enhanced cytokinin levels in ipt transgenic tobacco. In: Kaminek M, Mok DWS and Zazimalova E (eds), Physiology and Biochemistry of Cytokinins in Plants. (pp. 77–82) SPB Academic Publishing, The Hague, The Netherlands.
Bernier G (1988) The control of floral evocation and morphogenesis. Annu Rev Plant Physiol 39: 175–219.
Besnard-Wilbaut C (1981) Effectiveness of gibberellins and 6-benzyladenine on flowering of Arabidopsis thaliana. Physiol Plant 53: 205–212.
Chen CM and Melitz DK (1979) Cytokinin biosynthesis in a cell-free system from cytokinin-autotrophic tobacco tissue cultures. FEBS Lett 107: 15–20.
Chrirgwin JM and Mac Donald RJ (1979) Isolation of the total RNA from plants. Biochemistry 18: 5294–5299.
Estruch JJ, Prinsen E, Van Onckelen H, Schell J and Spena A (1991) Viviparous leaves produced by somatic activation of an inactive cytokinin-synthesizing gene. Science 254: 1364–1367.
Estruch JJ, Granell A, Hansen G, Prinsen E, Redig P, Van Onckelen H, Schwarz-Sommer Z, Sommer H and Spena A (1993) Floral development and expression of floral homeotic genes are influenced by cytokinins. Plant J 4: 379–384.
Faiss M, Zalubilova J, Strnad M and Schmulling T (1997) Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J 12: 401–415.
Finkelstein RR, Estelle MA, Martinez-Zapater JM and Somerville CR (1987) Arabidopsis as a tool for the identification of genes involved in plant development. In: Goldberg RB and Verma DP (eds), Temporal and Spatial Regulation of Plant Genes. (pp. 37–48) Springer Verlag, New York.
Gan S and Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270: 1986–1988.
Horgan R (1992) Present and future prospects for cytokinin research. In: Kaminek M, Mok DWS and Zazimalova E (eds), Physiology and Biochemistry of Cytokinins in Plants. (pp. 3–14) SPB Academic Publishing, The Hague, The Netherlands.
King P (1988) Plant hormone mutants. Trends Genet 4: 157–162.
Klee HJ, Horsch RB and Rogers SG (1987) Agrobacterium-mediated plant transformation and its further applications to plant biology. Annu Rev Plant Physiol 38: 467–486.
Koltunow AM, Truettner J, Cox KH, Wallroth M and Goldberg RB (1990) Different temporal and spatial gene expression paterns occur during anther development. Plant Cell 2: 1201–1224.
Kriete G, Niehaus K, Perlick AM and Puhler A (1996) Male sterility in transgenic tobacco plants induced by tapetum-specific deacetylation of the externally applied non-toxic compound N-acetyl-L-phosphinothricin. Plant J 9: 809–818.
Laloue M and Pethe C(1982) Dynamics of cytokinin metabolism in tobacco cells. In: Wareing FP (ed.), Plant Growth Substances. (pp. 185–195) Academic Press, London.
Ma M, Zhou DF, Guo Y, Kuang TY, Tang PS and Lin ZP (1996) The expression of GUS gene driven by T-cyt promoter in transgenic tobacco and potato. Acta Botan. Sin. 38: 169–173.
Mariani C, Beuckeleer MD, Truettner J, Leemans J and Goldberg RB (1990) Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 347: 737–741.
Mariani C, Gossele M, De Beuckeleer M, De Block M, Goldberg RB, De Greef W and Leemans J (1992) A chimeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature 357: 384–387.
Martineau B, Houck CK, Sheehy RE and Hiatt WR (1994) Fruit-specific expression of the A. tumefaciens isopentenyl transferase gene in tomato: effects on fruit ripening and defence-related gene expression in leaves. Plant J 5: 11–19.
McGaw BA, Scott IM and Horgan R (1984) Cytokinin biosynthesis and metabolism. In: Crozier A and Hillman JR (eds), The Biosynthesis and Metabolism of Plant Hormones. (pp. 105–133) Cambridge University Press, Cambridge.
McGaw BA (1987) Cytokinin biosynthesis and metabolism In: Davies PJ (ed.), Plant Hormones and Their Role in Plant Growth and Development. (pp.76–93) Martinus Nijhoff, Dordrecht, The Netherlands.
McKenzie MJ, Mett V, Reynolds PHS and Jameson PE (1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol 116: 969–977.
Medford JI, Horgan R, El-Sawi Z and Klee HJ (1989) Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell 1: 403–413.
Michniewicz M and Kamienska A (1965) Flower formation induced by kinetin and vitamin E treatment in long-day plant (Arabidopsis thaliana) grown in short day. Naturwissenschaften 52: 623.
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497.
Porebski S, Bailey G and Baum BR (1997) Modification of CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15: 8–15.
Redig P, Schmulling T and Van Onckelen H (1996) Analysis of cytokinin metabolism in ipt transgenic tobacco by liquid chromatography-tandem mass spectrometry. Plant Physiol 122: 141–148.
Sambrook J, Fritsch EF and Mariatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York.
Sembdner G, Atzorn R and Schneider G (1994) Plant hormone conjugation. Plant Mol Biol 26: 1459–1481.
Schmulling T, Beinsberger S, De Greef J, Schell J, Van Onckelen H and Spena A (1989) Construction of a heat-inducible chimeric gene to increase the cytokinin content in transgenic plant tissue. FEBS Lett 249: 401–406.
Smart CM, Scofield SR, Bevan MW and Dyer TA (1991) Delayed leaf senescence in tobacco plants transformed with tmr, a gene for cytokinin production in Agrobacterium. Plant Cell 3: 647–656.
Smigocki AC (1991) Cytokinin content and tissue distribution in plants transformed by a reconstructed isopentenyl transferase gene. Plant Mol Biol 16: 105–115.
Smigocki AC, Neal JW, McCanna I, Douglass L (1993) Cytokinin-mediated insect resistance in Nicotiana plants transformed with the ipt gene. Plant Mol Biol 23: 325–335.
Van Loven K, Beinsberger SEI, Valcke RLM, Van Onckelen HA and Clijsters HMM (1993) Morphometric analysis of the growth of Phsp70-ipt transgenic tobaco plants. J Exp Bot 44: 101–109.
Wareing, PF and Phillips IDJ (1981) Growth and Differentiation in Plants. Pergamon Press, New York.
Wullems GJ, Molendijk L, Ooms G and Schilperoort RA (1981) Retention of tumor markers in F1 progeny plants from in vitro induced octopine and nopaline tumor tissues. Cell 24: 719–727.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sa, G., Mi, M., He-Chun, Y. et al. Anther-specific expression of ipt gene in transgenic tobacco and its effect on plant development. Transgenic Res 11, 269–278 (2002). https://doi.org/10.1023/A:1015692127101
Issue Date:
DOI: https://doi.org/10.1023/A:1015692127101