Abstract
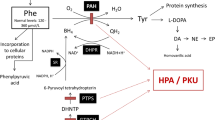
Hereditary tyrosinaemia type I (HT I) (McKusick 276700) is caused by a deficiency of fumarylacetoacetate hydrolase (FAH) activity, the last enzyme in the tyrosine catabolic pathway. Homozygous disruption of the gene encoding FAH in mice (Fah) causes neonatal lethality (i.e. lethal Albino deletion c14CoS mice), which limits the use of this animal as a model for HT I. We developed a new mouse model that carries two genetic defects, Fah and 4-hydroxyphenyl-pyruvate dioxygenase (Hpd). The double mutant Fah−/−Hpd−/− mice grew normally without evidence of liver and renal disease, showing a phenotype similar to Hpd−/− mice. Complete blockage of the tyrosine catabolic pathway at the, step of HPD prevents development of clinical phenotypes. Administration of homogentisate resulted in rapid apoptosis of hepatocytes and renal tubular epithelial cells, a central feature of visceral injury in patients with HT I. Simultaneously, renal tubular function was impaired, resulting in Fanconi syndrome. Apoptosis of hepatocyte and renal tubular cells is prevented by the caspase inhibitors YVAD or DEVD. However, these inhibitors do not prevent the release of cytochrome c or the development of renal tubular dysfunction. Apoptosis of hepatocytes and of renal tubular epithelial cells are characteristic features of this disease and the apoptotic signal in this disease seems to be initiated by fumarylacetoacetate.
Similar content being viewed by others
REFERENCES
Awata H, Endo F, Tanoue A, Kitano A, Nakano Y, Matsuda I (1994) Structural organization and analysis of the human fumarylacetoacetate hydrolase gene in tyrosinemia type I. Biochim Biophys Acta 1226: 168–172.
Berger R, Smit GP, Stoker-de Varies SA, et al (1981) Deficiency of fumarylacetoacetase in a patient with hereditary tyrosinemia. Clin Chim Acta 114: 37–44.
Endo F, Katoh H, Yamamoto S, Matsuda I (1991) A murine model for type III tyrosinemia: lack of immunologically detectable 4-hydroxyphenylpyruvic acid dioxygenase enzyme protein in a novel mouse strain with hypertyrosinemia. Am J Hum Genet 48: 704–709.
Endo F, Awata H, Katoh H, Matsuda I (1995) A nonsense mutation in the 4-hydroxyphenylpyruvic acid dioxygenase gene (Hpd) causes skipping of the constitutive exon and hypertyrosinemia in mouse strain III. Genomics 25: 164–169.
Endo F, Kubo S, Awata H, et al (1997) Complete rescue of lethal albino c14CoS mice by null mutation of 4-hydroxyphenylpyruvate dioxygenase and induction of apoptosis of hepatocytes in these mice by in vivo retrieval of the tyrosine catabolic pathway. J Biol Chem 272: 24426–24432.
Gluecksohn-Waelsch S (1979) Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell 16: 225–237.
Grompe M, Al-Dhalimy M, Finegold M, et al (1993) Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 7: 2298–2307.
Grompe M, Lindstedt S, Al-Dhalimy M, Kennaway NG (1995) Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinemia type I. Nature Genetics 10: 453–460.
Kelsey G, Ruppert S, Beermann F, Grund C, Tanguay M, Schutz G (1993) Rescue of mice homozygous for lethal albino deletions: implications for an animal model for the human liver disease tyrosinemia type I. Genes Dev 7: 2285–2297.
Kivittingen EA, Jellum E, Stokke O (1981) Assay of fumarylacetoacete famarylhydrolase in human liver: deficient activity in a case of hereditary tyrosinemia. Clin Chim Acta 115: 311–319.
Klebig ML, Russell LB, Rinchik EM (1992) Murine fumarylacetoacetate hydrolase (Fah) gene is disrupted by a neonatally lethal albino deletion that defines the hepatocyte-specific developmental regulation 1 (hsdr-1) locus. Proc Natl Acad Sci USA 89: 1363–1367.
Kubo S, Kiwaki K, Awata H, et al (1997) In vivo correction with recombinant adenovirus of 4-hydroxyphenylpyruvic acid dioxygenase deficiencies in strain III mice. Hum Gene Ther 8: 65–71.
Kubo S, Sun MS, MyaharaM, et al (1998) Hepatocyte injury in tyrosinemia type 1 is induced by fumarylacetoacetate and is inhibited by caspase inhibitors. Proc Natl Acad Sci USA 95: 9552–9557.
Lindblad B, Lindstedt S, Steen G (1977) On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci USA 74: 4641–4645.
Lindstedt S, Holme E, Lock EA, Hjalmarson, O, Strandvik B (1992) Treatment of hereditary tyrosinemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340: 813–817.
Mitchell G, Larochelle J, Lambert M, et al (1990) Neurologic crises in hereditary tyrosinemia. N Engl J Med 322: 432–437.
Mitchell GA, Lambert M, Tanguay RM (1995) Hypertyrosinemia. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease, 7th edn. New York: McGraw-Hill, 1077–1106.
Phaneuf D, Lambert M, Laframboise R, Mitchell G, Lettre F, Tanguay RM (1992) Type 1 hereditary tyrosinemia: evidence for molecular heterogeneity and identification of a causal mutation in a French Canadian patient. J Clin Invest 90: 1185–1192.
Ruppert S, Bothart M, Bosch F, Schmid W, Foumier REK, Schutz G (1990) Two genetically defined trans-acting loci coordinately regulate overlapping sets of liver-specific genes. Cell 61: 895–904.
Ruppert S, Kelsey G, Schedl A, Schmid E, Thies E, Schutz, G (1992) Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev 6: 1430–1443.
Sakai K, Kitagawa T (1957) An atypical case of tyrosinosis. Part 1. Jikei Med J 2: 1–10.
Sun MS, Hattori S, Kubo S, Awata H, Matsuda I, Endo F (2000) A mouse model of renal tubular injury of tyrosinemia type 1: development of de Toni Fanconi syndrome and apoptosis of renal tubular cells in Fah/Hpd double mutant mice. J Am Soc Nephrol 111: 291–300.
Trigg MJ, Gluecksohn-Waelsch S (1973) Ultrastructural basis of biochemical effects in a series of lethal alleles in the mouse. J Cell Biol 58: 549–563.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endo, F., Sun, MS. Tyrosinaemia type I and apoptosis of hepatocytes and renal tubular cells. J Inherit Metab Dis 25, 227–234 (2002). https://doi.org/10.1023/A:1015646400182
Issue Date:
DOI: https://doi.org/10.1023/A:1015646400182