Abstract
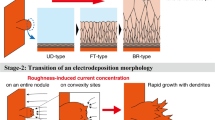
The galvanostatic technique on a laboratory scale has been shown to be a useful tool in detecting the presence of nodules on the cathode during copper electrodeposition by using the value of the starting electrolytic potential and by the presence of a cathodic polarization peak on the potential–time curve. Studying the morphology of the deposit with a scanning electron microscope at various magnifications confirmed the galvanostatic results. It is postulated that inappropriate concentrations and/or ratios of the additives (thiourea, gelatin and chloride ions) are associated with a certain current density that generates intergranular microcracks due to adsorption of the additives and leads to the formation of nodules at the macroscale.
Similar content being viewed by others
References
D.F. Suarez and F.A. Olson, J. Appl. Electrochem. 22 (1992) 1002.
E. Ilgar, Ph.D thesis, University of Missouri (1993).
C.T. Wang and T.J. O'Keefe, in P.E. Richardson, S. Srinivasan and R. Woods (Eds), Proceedings of the International Symposium on 'Electrochemistry in Mineral and Metal Processing' (Electrochemical Society Pennington, NJ, 1984) p. 655.
O. Forsen, in Copper '90, 'Refining, Fabrication, Markets' (Institute of Metals, Västeräs, Sweden, 1–3 Oct. (1990), p. 189.
O. Forsen, A. Kotzschmar and A.E. Antila, Galvanotechnick 86 (1995) 3580.
L. Pajdowski, 'Problems and Methods of Coordination Chemistry' (Institute of Chemistry, University of Wroclaw, Wroclaw, Poland, 1981).
R. Winand, Hydrometallurgy 29 (1994) 56.
Z. Gorlich, L. Blaz and A. Diazon, Non Ferrous Ores and Metals 22 (1977) 522.
L. Mirkova, N. Petkova, I. Popova and St. Rashkov, Hydrometallurgy 36 (1994) 201.
H. Fisher, 'Elektrolytische abscheidung und Elektrokristallisation von Metallen' (Springer, Berlin, 1954).
B. Veilleux, A-M. Lafront and E. Ghali, J Appl. Electrochem, in press.
C. De Maere and R. Winand, in W.C. Cooper, D.B. Dreisinger, J.E. Dutrizac, H. Hein and G. Ugarte (Eds), Proc. Copper 95 – Cobre 95 International Conference, Vol. III, 'Electrore.ning and Hydrometallurgy of Copper' (Metallurgical Society of CIM, Montreal, Canada, 1995), p. 267.
T. Pearson and J.K. Dennis, Surf. Coat Technol. 42 (1990) 69.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lafront, AM., Veilleux, B. & Ghali, E. Galvanostatic and microscopic studies of nodulation during copper electrolysis. Journal of Applied Electrochemistry 32, 329–337 (2002). https://doi.org/10.1023/A:1015589725641
Issue Date:
DOI: https://doi.org/10.1023/A:1015589725641