Abstract
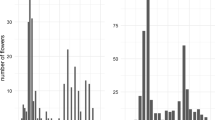
Flowering phenology is often strongly constrained by phylogenetichistory: many closely-related plants have very similar phenologies. On theotherhand, divergent flowering phenologies can function as isolating mechanisms,which may be reinforced if related plants occur sympatrically. I investigatedflowering phenology and reproductive output of sister species of barrel cacti,Ferocactus cylindraceus and F.wislizeni, where they occur sympatrically in the Sonoran Desertsurrounding Tucson, Arizona. Ferocactus cylindraceus beganblooming in May, and continued until early or mid-October, with a bimodalpattern of flowering amplitude. Individuals in the study population weremoderately well-synchronized phenologically. Ferocactuswislizeni began blooming in July, and also continued until early ormid-October, with a single peak of intensity; individuals in the studypopulation were well- synchronized phenologically. In both species, the vastmajority of individuals bloom every year. Plant size was positively correlatedwith flowering amplitude in both species, and with flowering onset inF. wislizeni. The study population of F.cylindraceus was strongly affected by a flower-eating caterpillar inall years, with the earliest flowers most likely to be destroyed. ForF. wislizeni, seed number per fruit was highest forflowersopen in the middle of the blooming season in 1998. Other components ofindividual plant phenology, including among-plant synchrony, had littleinfluence on reproductive output.
Similar content being viewed by others
References
Augspurger C.K. 1983. Phenology, flowering synchrony, and fruit set of six neotropical shrubs. Biotropica 15: 257–267.
Benson L.D. 1982. The cacti of the United States and Canada Stanford University Press, Stanford.
Bishop J.G. and Schemske D.W. 1998. Variation in flowering phenology and its consequences for lupines colonizing Mount St. Helens. Ecology 79: 534–546.
Bowers J.E. 1994. Natural conditions for seedling emergence of three woody species in the northern Sonoran Desert. Madroño 41: 73–84.
Bowers J.E. and Dimmitt M.A. 1994. Flowering phenology of six woody plants in the northern Sonoran Desert. Bull. Torrey Bot. Club 121: 215–229.
Bronstein J.L. 1995. The plant-pollinator landscape. In: Hansson L., Fahrig L. and Merriam G. (eds), Mosaic Landscapes and Ecological Processes. Chapman & Hall, London, pp. 256–288.
Clark J.S., Beckage B., Camill P., Cleveland B., HilleRisLambers J., Lichter J. et al. 1999. Interpreting recruitment limitation in forests. Am. J. Bot 86: 1–16.
Cota J.H. and Wallace R.S. 1997. Chloroplast DNA evidence for divergence in Ferocactus and its relationships to North American columnar cacti (Cactaceae: Cactoideae). Syst. Bot. 22: 529–542.
Grant V. and Grant K.A. 1979. Pollination of Echinocereus fasciculatus and Ferocactus wislizenii. Plant Syst. Evol. 132: 85–90.
Haddock R.C. and Chaplin S.J. 1982. Pollination and seed production in two phenologically divergent prairie legumes (Baptisia leucophaea and B. leucantha). Am. Midland Nat. 108: 175–186.
Jordan P.W. and Nobel P.S. 1981. Seedling establishment of Ferocactus acanthodes in relation to drought. Ecology 62: 901–906.
Kearney T.H. and Peebles R.H. 1951. Arizona Flora University of California Press, Berkeley.
Kochmer J.P. and Handel S.N. 1986. Constraints and competition in the evolution of flowering phenology. Ecol. Monogr. 56: 303–325.
Madeira J.A. and Fernandes G.W. 1999. Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil. J. Tropical Ecol. 15: 463–479.
McIntosh M.E. 2001. Plant size, breeding system, and limits to re-productive success in two sister species of Ferocactus (Cacta-ceae). Plant Ecol.
Newstrom L.E., Frankie G.W., Baker H.G. and Colwell R.K. 1994. Diversity of long-term flowering patterns. In: McDade L.A., Bawa K.S., Hespenheide H.A. and Hartshorn G.S. (eds), La Selva: Ecology and Natural History of a Neotropical Rain Forest. University of Chicago Press, Chicago, pp. 142–160.
Ollerton J. and Diaz A. 1999. Evidence for stabilising selection acting on flowering time in Arum maculatum (Araceae): the influence of phylogeny on adaptation. Oecologia 119: 340–348.
Ollerton J. and Lack A.J. 1992. Flowering phenology: an example of relaxation of natural selection? Trends Ecol. Evol. 7: 274–276.
Ollerton J. and Lack A. 1998. Relationships between flowering phenology, plant size and reproductive success in Lotus corniculatus (Fabaceae). Plant Ecol. 139: 35–47.
O'Neil P. 1997. Natural selection on genetically correlated phenological characters in Lythrum salicaria L. (Lythraceae). Evolution 51: 267–274.
Pickering C.M. 1995. Variation in flowering parameters within and among five species of Australian alpine Ranunculus. Aust. J. Bot. 43: 103–112.
Pierson E.A. and Turner R.M. 1998. An 85-year study of saguaro (Carnegiea gigantea) demography. Ecology 79: 2676–2693.
Primack R.B. 1980. Variation in the phenology of natural populations of montane shrubs in New Zealand. J. Ecol. 68: 849–862.
Primack R.B. 1987. Relationships among flowers, fruits, and seeds. Annu. Rev. Ecol. Syst. 18: 409–430.
Proença C.E.B. and Gibbs P.E. 1994. Reproductive biology of eight sympatric Myrtaceae from Central Brazil. New Phyt. 126: 343–354.
Rathcke B. and Lacey E.P. 1985. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 16: 179–214.
Romero-Schmidt H.L., Vega-Villasante F., Nolasco H. and Montaño C. 1992. The effect of darkness, freezing, acidity and salinity on seed germination of Ferocactus peninsulae (Cactaceae). J. Arid Environ. 23: 389–395.
SAS Institute Inc. 1989-99a. JMP IN® SAS Institute Inc., Cary, version 3.2.1.
SAS Institute, Inc. 1989-99b. JMP® start statistics Duxbury Press, Belmont.
Schmitt J. 1983. Individual flowering phenology, plant size, and reproductive success in Linanthus androsaceus, a California annual. Oecologia 59: 135–140.
Shreve F. and Wiggins I.L. 1964. Vegetation and Flora of the Sonoran Desert Vol. 12. Stanford University Press, Stanford.
Soliva M. and Widmer A. 1999. Genetic and floral divergence among sympatric populations of Gymnadenia conopsea S.L. (Orchidaceae) with different flowering phenology. Int. J. Plant Sci. 160: 897–905.
Stephenson A.G. 1981. Flower and fruit abortion: proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 12: 253–279.
Turner R.M., Bowers J.E. and Burgess T.L. 1995. Sonoran Desert Plants: An Ecological Atlas University of Arizona Press, Tucson.
Wright S.J. and Calderon O. 1995. Phylogenetic patterns among tropical flowering phenologies. J. Ecol. 83: 937–948.
Zar J.H. 1996. Biostatistical Analysis 3rd edn. Prentice-Hall, Englewood Cliffs, NJ.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McIntosh, M.E. Flowering phenology and reproductive output in two sister species of Ferocactus (Cactaceae). Plant Ecology 159, 1–13 (2002). https://doi.org/10.1023/A:1015589002987
Issue Date:
DOI: https://doi.org/10.1023/A:1015589002987