Abstract
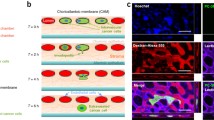
Tumor cells acquire the ability to enter blood vessels surrounding the primary tumor, endowing them with the capacity to disseminate and become established in distant sites, originating a metastasis. Determination of the intravasation ability of tumor cells is thus important, as it can be correlated with their potential malignancy. To analyze the intravasation phenotype of human tumor cells in vivo, we performed chick embryo chorioallantoic membrane (CAM) assays. Cells were inoculated on the CAM of 9-day-old chick embryos and the membrane at the opposite side of the egg was recovered after 48 h incubation. To measure intravasation ability, we calculated the amount of human DNA in each CAM sample by real-time PCR of Alu sequences and SYBR Green I fluorescence detection. This analysis showed a detection limit of 1 human cell per 105 total cells, and we were able to distinguish between tumor cells of distinct invasive capacity. This assay has several advantages over current methods to measure intravasation ability, including the elimination of post-PCR analysis, sensitivity and easy scale-up of sample numbers.
Similar content being viewed by others
References
Liotta L, Stracke M. Tumor invasion and metastasis: Biochemical mechanisms.Cancer Treat Res1988;40:223–38.
Kohn E, Liotta L. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res 1995;55:1856–62.
Quigley J, Armstrong P. Tumor cell intravasation Alu-cidated: The chick embryo opens the window.Cell 1998;94:281–4.
Zhang L, Kharbanda S, McLeskey S et al. Overexpression of fi-broblasts growth factor 1 in MCF-7 breast cancer cells facilitates tumor cell dissemination but does not support the development of macrometastases in the lungs or lymph nodes.Cancer Res1999;59: 5023–9.
Hall S, Thompson T. Spontaneous but not experimental metastasis activities differentiate primary tumor-derived vs. metastasis-derived mouse prostate cancer cell lines.Clin Exp Metastasis1997;15:630–8.
Carlson BM. Patten's Foundations of Embryology.New York: McGraw-Hill 1988.
Cao R, Farnebo J, Kurimoto M et al. Interleukin-18 acts as an angiogenesis and tumor suppressor.FASEB J1999;13:2195–202.
Jia L, Wu C, Guo W et al. Antiangiogenic effects of S-nitrosocaptopril crystals as a nitric oxide donor.Eur J Pharmacol2000;391:137–44.
Vacca A, Iurlaro M, Ribatti D et al. Antiangiogenesis is produced by nontoxic doses of vinblastine.Blood1999;94:4143–55.
Jiang B, Zheng J, Aoki M et al. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells.Proc Natl Acad Sci USA2000;97: 1749–53.
Ribatti D, Leali D, Vacca A et al. In vivo angiogenic activity of urokinase: Role of endogenous fibroblast growth factor-2.J Cell Sci1999; 112:4213–21.
Lyu MA, Choi YK, Park BN et al. Over-expression of urokinase receptor in human epidermoid-carcinoma cell line (HEp3) increases tumorigenicity on chorioallantoic membrane and in severe-combinedimmunodeficient mice.Int J Cancer1998;77:257–63.
Haeckel C, Krueger S, Roessner A. Antisense inhibition of urokinase: effect on malignancy in a human osteosarcoma cell line.Int J Cancer 1998; 77: 153–60.
Kobayashi T, Koshida K, Endo Y et al. A chick embryo model for metastatic human prostate cancer.Eur Urol1998;34:154–60.
Brooks P, Lin JM, French D et al.Subtractive immunization yields monoclonal antibodies that specifically inhibit metastasis.J Cell Biol 1993;122:1351–9.
Yamamoto H, Endo Y, Nomura M et al. Assessment of the metastatic ability of rat hepatoma cells in chick embryos by the polymerasechain reaction.Anticancer Res1996;16:413–8.
Kim J, Yu W, Kovalsky K et al. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay.Cell 1998;94:353–62.
Adams RLP. Cell Culture for Biochemists.Amsterdam: Elsevier Science Publishers 1990.
Billstrom A, Lecander I, Dagnaes-Hansen F et al. Differential expression of uPA in an aggressive (DU-145) and a nonaggressive (1013L) human prostate cancer xenograft.Prostate1995;26:94–104.
Knox J, Mack C, Powell W et al. Prostate tumor cell invasion: A comparison of orthotopic and ectopic models.Invasion Metastasis1993; 13:325–31.
Mira E, Lacalle R, González MA et al. A role for chemokine receptor transactivation in growth factor signaling.EMBO Rep2001;2:151–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mira, E., Lacalle, R.A., Gómez-Moutón, C. et al. Quantitative determination of tumor cell intravasation in a real-time polymerase chain reaction-based assay. Clin Exp Metastasis 19, 313–318 (2002). https://doi.org/10.1023/A:1015563031769
Issue Date:
DOI: https://doi.org/10.1023/A:1015563031769