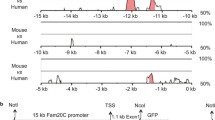
Abstract
BMP-7, a member of the bone morphogenetic protein subfamily of the TGFβ-superfamily is highly expressed in the murine kidney. BMP-7 is involved in fetal nephron development and mesenchymal to epithelial cell differentiation. Constitutive BMP-7 expression is found in tubular and glomerular epithelial cells of the adult kidney. BMP-7 may play a role in physiology and pathophysiology of the adult kidney since BMP-7 gene expression in acute renal ischemia is diminished and injection of recombinant BMP-7 into rats with ischemic acute renal failure preserves renal function. In order to investigate the transcriptional regulation of BMP-7, this study was undertaken to clone and characterize the promoter of the murine BMP-7 gene. A 1394 bp sequence of the 5′-flanking region of the BMP-7 gene was isolated and subcloned. No TATA and CAAT box consensus motifs could be identified as shown for promoters of other BMPs. Using in vitro transfection assays, the 5′-flanking region revealed moderate to strong basal promoter activity. PMA increased basal BMP-7 promoter activity. Thus BMP-7 gene transcription might involve at least in part a PKC-dependent pathway. The cloning of a 5′-flanking region of the BMP-7 gene should provide a useful tool for future studies on the transcriptional regulation of BMP-7 gene expression.
Similar content being viewed by others
References
Massague J: The transforming growth factor-beta family. Annu Rev Cell Biol 6: 597–641, 1990
Ying SY: Inhibins, activins and follistatins. J Steroid Biochem 33: 705–713, 1989
Lyons KM, Jones CM, Hogan BL: The DVR gene family in embryonic development. Trends Genet 7: 408–412, 1991
Sporn MB, Roberts AB: Transforming growth factor-beta: Recent progress and new challenges. J Cell Biol 119: 1017–1021, 1992
Urist MR: Bone: Formation by autoinduction. Science 150: 893–899, 1965
Sampath TK, Reddi AH: Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA 78: 7599–7603, 1981
Urist MR, Huo YK, Brownell AG, Hohl WM, Buyske J, Lietze A, Tempst P, Hunkapiller M, DeLange RJ: Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci USA 81: 371–375, 1984
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA: Novel regulators of bone formation: Molecular clones and activities. Science 242: 1528–1534, 1988
Vukicevic S, Helder MN, Luyten FP: Developing human lung and kidney are major sites for synthesis of bone morphogenetic protein-3 (osteogenin). J Histochem Cytochem 42: 869–875, 1994
Takahashi H, Ikeda T: Transcripts for two members of the transforming growth factor-beta superfamily BMP-3 and BMP-7 are expressed in developing rat embryos. Dev Dyn 207: 439–449, 1996
Suzuki A, Nishimatsu S, Murakami K, Ueno N: Differential expression of Xenopus BMPs in early embryos and tissues. Zoolog Sci 10: 175–178, 1993
Ozkaynak E, Schnegelsberg PN, Jin DF, Clifford GM, Warren FD, Drier EA, Oppermann H: Osteogenic protein-2. A new member of the transforming growth factor-beta superfamily expressed early in embryogenesis. J Biol Chem 267: 25220–25227, 1992
Lyons KM, Pelton RW, Hogan BL: Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109: 833–844, 1990
Lyons KM, Pelton RW, Hogan BL: Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-betalike genes coordinately regulate aspects of embryonic development. Genes Dev 3: 1657–1668, 1989
Knittel T, Fellmer P, Muller L, Ramadori G: Bone morphogenetic protein-6 is expressed in nonparenchymal liver cells and upregulated by transforming growth factor-beta 1. Exp Cell Res 232: 263–269, 1997
Jones CM, Lyons KM, Hogan BL: Involvement of bone morphogenetic protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111: 531–542, 1991
Helder MN, Ozkaynak E, Sampath KT, Luyten FP, Latin V, Oppermann H, Vukicevic S: Expression pattern of osteogenic protein-1 (bone morphogenetic protein-7) in human and mouse development. J Histochem Cytochem 43: 1035–1044, 1995
Lyons KM, Hogan BL, Robertson EJ: Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev 50: 71–83, 1995
Ozkaynak E, Schnegelsberg PN, Oppermann H: Murine osteogenic protein (OP-1): High levels of mRNA in kidney. Biochem Biophys Res Commun 179: 116–123, 1991
Dudley AT, Lyons KM, Robertson EJ: A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807, 1995
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G: BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820, 1995
Vukicevic S, Kopp JB, Luyten FP, Sampath TK: Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7). Proc Natl Acad Sci USA 93: 9021–9026, 1996
Dudley AT, Godin RE, Robertson EJ: Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13: 1601–1613, 1999
Godin RE, Takaesu NT, Robertson EJ, Dudley AT: Regulation of BMP7 expression during kidney development. Development 125: 3473–3482, 1998
Bhandari B, Beckwith KD, Miller RE: Cloning, nucleotide sequence, and potential regulatory elements of the glutamine synthetase gene from murine 3T3-L1 adipocytes. Proc Natl Acad Sci USA 85: 5789–5793, 1988
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467, 1977
Yee J, Kuncio GS, Bhandari B, Shihab FS, Neilson EG: Identification of promoter activity and differential expression of transcripts encoding the murine stromelysin-1 gene in renal cells. Kidney Int 52: 120–129, 1997
Herzlinger D, Koseki C, Mikawa T, al-Awqati Q: Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development 114: 565–572, 1992
Simon M, Maresh JG, Harris SE, Hernandez JD, Arar M, Olson MS, Abboud HE: Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am J Physiol 276: F382–F389, 1999
Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, Podkolodny NL, Kolchanov NA: Databases on transcriptional regulation: TRANSFAC, TRRD, and COMPEL. Nucleic Acids Res 26: 364–370, 1998
Sugiura T: Cloning and functional characterization of the 5′-flanking region of the human bone morphogenetic protein-2 gene. Biochem J 338: 433–440, 1999
Hino J, Takao M, Takeshita N, Konno Y, Nishizawa T, Matsuo H, Kangawa K: cDNA cloning and genomic structure of human bone morphogenetic protein-3B (BMP-3b). Biochem Biophys Res Commun 223: 304–310, 1996
Shore EM, Xu M, Shah PB, Janoff HB, Hahn GV, Deardorff MA, Sovinsky L, Spinner NB, Zasloff MA, Wozney JM, Kaplan FS: The human bone morphogenetic protein 4 (BMP-4) gene: Molecular structure and transcriptional regulation. Calcif Tissue Int 63: 221–229, 1998
Tamada H, Kitazawa R, Gohji K, Kamidono S, Maeda S, Kitazawa S: Molecular cloning and analysis of the 5′-flanking region of the human bone morphogenetic protein-6 (BMP-6). Biochim Biophys Acta 1395: 247–251, 1998
Sakaue M, Kitazawa S, Nishida K, Kitazawa R, Maeda S: Molecular cloning and characterization of human bone morphogenic protein (BMP)-5 gene promoter. Biochem Biophys Res Commun 221: 768–772, 1996
Kitten AM, Kreisberg JI, Olson MS: Expression of osteogenic protein-1 mRNA in cultured kidney cells. J Cell Physiol 181: 410–415, 1999
Pyke C, Kristensen P, Ostergaard PB, Oturai PS, Romer J: Proteoglycan expression in the normal rat kidney. Nephron 77: 461–470, 1997
Horowitz A, Simons M: Phosphorylation of the cytoplasmic tail of syndecan-4 regulates activation of protein kinase Calpha. J Biol Chem 273: 25548–25551, 1998
Oh ES, Woods A, Lim ST, Theibert AW, Couchman JR: Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4,5-bisphosphate coordinately regulate protein kinase C activity. J Biol Chem 273: 10624–10629, 1998
Chen RH, Ding WV, McCormick F: Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem 275: 17894–17899, 2000
Cadigan KM, Nusse R: Wnt signaling: A common theme in animal development. Genes Dev 11: 3286–3305, 1997
van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H: Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799, 1997
Rights and permissions
About this article
Cite this article
Simon, M., Feliers, D., Arar, M. et al. Cloning of the 5′-flanking region of the murine bone morphogenetic protein-7 gene. Mol Cell Biochem 233, 31–37 (2002). https://doi.org/10.1023/A:1015546615027
Issue Date:
DOI: https://doi.org/10.1023/A:1015546615027