Abstract
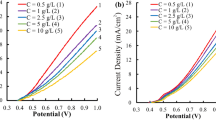
Improvement of the conventional silver oxide electrode has been achieved by mixing with a certain amount of nanophase Ag2O particles. The periodic charge–discharge results of various electrode compositions under different discharge conditions (e.g., 1C, C/3 and C/10) provides a clear comparison of electrochemical performance. When the nanophase Ag2O species are between 10% and 35% of the active total amount, an extra 20–30% discharge capacity can be expected. However, if the electrode consists only of nanophase Ag2O as active material, it does not have effective charge–discharge cycles. The favourable role of nanophase Ag2O in a silver oxide electrode is attributed to the increased active material utilization because of optimum mass and charge transfer.
Similar content being viewed by others
References
C.P. Wales, J. Electrochem. Soc. 108 (1961) 395.
C.P. Wales and J. Burbant, J. Electrochem. Soc. 111 (1964) 1002.
C.K. Dyerand T.P. Hoar, Electrochim. Acta 20 (1975) 161.
V. Lazarescu, O. Radovici and M. Vass, Electrochim. Acta 30 (1985) 1407.
G.S. Popkirov, M. Burmeister and R.N. Schimdler, J. Electroanal. Chem. 380 (1995) 249.
S.L. Chen, B.L. Wu and C.S. Cha, J. Electroanal. Chem. 416 (1996) 53.
W.S. Gra. and H.H. Stadelmaier, J. Electrochem. Soc. 105 (1958) 446.
J. Ambrose and R.G. Barradas, Electrochim. Acta 19 (1974) 781.
J.M.M. Droog and T. Huisman, J. Electroanal. Chem. 115 (1980) 211.
M. Lopez Teijelo, J.R. Vilche and A.J. Arvia, J. Electroanal. Chem. 162 (1984) 207.
N.A. Kotov, I. Dekany and J.H. Fendller, J. Phys. Chem. 99 (1995) 13065.
Q.W. Li, J. Li, X. Xia and Y.L. Chao, Chinese J. Chem. 57 (1999) 491.
H.T. Liu and X. Xia, Chinese J. Chem. 58 (2000) 992.
C.P. Wales and J. Burbant, J. Electrochem. Soc. 106 (1959) 885.
A. Fleischerand J.J. Lander (Eds), 'Zinc–Silver Oxide Batteries' (J. Wiley & Sons, New York, 1971).
K. Takeda and T. Hattori, J. Electrochem. Soc. 146 (1999) 3190.
T.P. Dirkse, J. Electrochem. Soc. 109 (1962) 173.
C.P. Wales and J. Burban, J. Electrochem. Soc. 112 (1965) 13.
T.P. Dirkse and B. Wiers, J. Electrochem. Soc. 106 (1959) 284.
R.E.F. Einerhand, W. Visscher, J.J.M. de Goeij and E. Barendrecht, J. Electrochem. Soc. 138 (1991) 1.
X. Xia and C.L. Meng, Chin. J. Appl. Chem. 15 (1998) 13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Xia, X. & Guo, Z. A novel silver oxide electrode and its charge–discharge performance. Journal of Applied Electrochemistry 32, 275–279 (2002). https://doi.org/10.1023/A:1015541703258
Issue Date:
DOI: https://doi.org/10.1023/A:1015541703258