Abstract
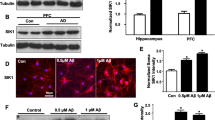
In this study we investigated the effects of the branched chain α-ketoacids accumulating in maple syrup urine disease (MSUD) on the concentrations of the high molecular weight neurofilament subunit (NF-H) associated with the cytoskeletal fraction of the cerebral cortex of 12-day-old rats. Cortical slices were incubated with α-ketoisocaproic acid (KIC), α-keto β-methylvaleric acid (KMV) and α-ketoisovaleric acid (KIV) at concentrations ranging from 0.5 to 1.0 mM. The cytoskeletal fraction was extracted and the immunoreactivity for phosphorylated and total NF-H was analyzed by immunoblotting. The in vitro 32P incorporation into NF-H was also determined. Results showed that treatment of tissue slices induced with KMV increased Triton-insoluble phosphorylated NF-H immunoreactivity, with no alteration in total NF-H immunoreactivity. Furthermore, KIC treatment drastically increased the total amount of NF-H, whereas KIV did not change either phosphorylated or total NF-H immunoreactivity. KMV also increased the in vitro 32P incorporation into NF-H, confirming the highly phosphorylated NF-H levels detected in the immunoblot. These findings demonstrate that KIC and KMV alter the dynamic regulation of NF-H assembly in the cytoskeletal fraction. Therefore we may suggest that cytoskeletal disorganization may be one of the factors associated with the neurodegeneration characteristic of MSUD disease.
Similar content being viewed by others
REFERENCES
Ackerley, S., Grierson, A.J., Brownlees, J., Thornhill, P., Anderton, B.H., Leigh, P.N., Shaw, C.E., and Miller, C.C.J. (2000). Glutamate slows axonal transport of neurofilaments in transfected neurons. J. Cell Biol. 150:165–176.
Archer, D.R., Watson, D.F., and Griffin, J.W. (1994). Phosphorylation-dependent immunoreactivity of neurofilaments and the rate of slow axonal transport in the central and peripheral axons of the rat dorsal root ganglion. J. Neurochem. 62:1119–1125.
Bignami, A., Chi, N.H., and Dahl, D. (1986). Neurofilament phosphorylation in peripheral nerve regeneration. Brain Res. 375:73–82.
Branco, T., Meirelles, R., Bevilaqua da Rocha, B., de Mattos-Dutra, A., Wajner, M., and Pessoa-Pureur, R. (2000). Alpha keto-isocaproate increases the in vitro 32P incorporation into intermediate filaments in cerebral cortex of rats. NeuroReport 11:3546–3550.
Carden, M.J., Trojanowski, J.Q., Schlaepfer, W.W., and Lee, V.M.-Y. (1987). Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J. Neurosci. 7:3489–3504.
Chuang, D.T., and Shih, V.E. (2001). Disorders of branched chain amino acid and keto acid metabolism. In (C.R. Scriver, A.L. Beaudet, W.L. Sly, and D. Valle, eds.), The Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill, New York, pp. 1971–2005.
deWaegh, S.M., Lee, V.M.-Y., and Brady, S.T. (1992). Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 68:451–463.
Draeger, U.C., and Hofbauer, A. (1984). Antibodies to heavy neurofilament subunit detect a subpopulation of damaged ganglion cells in retina. Nature 309:624–626.
Efron, M.L. (1965). Aminoaciduria. N. Engl. J. Med. 272:1058–1067.
Giasson, B.I., and Mushynski,W.E. (1997). Study of proline-directed protein kinases involved in phosphorylation of the heavy neurofilament subunit. J. Neurosci. 17:9466–9472.
Greenwood, J.Á, Troncoso, J.C., Costello, A.C., and Johnson, G.V.W. (1993). Phosphorylation modulates calpainmediated proteolysis and calmodulin binding of the 200 kDa and 160 kDa neurofilament proteins. J. Neurochem. 61:191–199.
Guidato, S., Bajaj, N.P., and Miller, C.C. (1996). Cellular phosphorylation of neurofilament heavy-chain by cyc dependent kinase-5 masks the epitope for monoclonal antibody N52. Neurosci. Lett. 217:157–160.
Hisanaga, S., Kusubata, M., Okumura, E., and Kishimoto, T. (1991). Phosphorylation of neurofilament H subunit at the tail domain by the CDC 2 kinase dissociates the association to microtubules. J. Biol. Chem. 266:21798–21803.
Hirano, A. (1991). Cytopathology of amyotrophic lateral sclerosis. In (L.P. Rowland, ed.), Advances in Neurology. Vol. 56. Amyotrophic lateral sclerosis and other motor neuron diseases. Raven, New York, pp. 91–101.
Hirokawa, N., Glicksman, M.A., and Willard, M.B. (1984). Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J. Cell Biol. 98:1523–1536.
Hoffmann, P.N., Griffin, J.W., and Price, D.L. (1984). Control of axonal caliber by neurofilament transport. J. Cell Biol. 99:705–714.
Hoffmann, P.N., Griffin, G.W., Gold, B.G., and Price, D.L. (1985). Slowing of neurofilament transport and the radial growth of developing nerve fibers. J. Neurosci. 5:2920–2929.
Jung, C., Yabe, J., Wang, F.-S., and Shea, T.B. (1998). Neurofilament subunits can undergo axonal transport without incorporation into Triton-insoluble structures. Cell Motil. Cytoskeleton 40:44–58.
Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 277:680–685.
Lee, M.K., and Cleveland, D.W. (1994). Neurofilament function and dysfunction: Involvement in axonal growth and neuronal disease. Curr. Opin. Cell Biol. 6:34–40.
Lowry, O.K., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–267.
Mattos, A.G., de Freitas, M.S., Camargo, M.M., and Pessoa-Pureur, R. (1994). Developmentally regulated in vitro phosphorylation of cytoskeletal proteins of the cerebral cortex of normal and malnourished rats. Dev. Neurosci. 16:38–43.
Nixon, R.A., Paskevich, P.A., Sihag, R.A., and Thayer, C.Y. (1994). Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: Influences on regional neurofilament accumulation, interneurofilament spacing, and axonal caliber. J. Cell Biol. 126:1031–1046.
Pant, H.C. (1988). Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 256:665–668.
Pollanen, M.S., Bergeron, C., and Weyer, L. (1994). Characterization of a shared epitope on cortical Lewy body fibrils and Alzheimer paired helical filaments. Acta Neuropathol. (Berl.) 88:1–6.
Schmidt, M.L., Martin, J.A., Lee, V.M.Y., and Trojanowski, J.Q. (1996). Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer's disease and Lewy body disorders. Acta Neuropathol. (Berl.) 91:475–481.
Schumacher, P.A., Eubanks, J.H., and Fehlings, M.G. (1999). Increased calpain I-mediated proteolysis, and preferential loss of dephosphorylated NF200, following traumatic spinal cord injury. Neuroscience 91:733–744.
Shea, T.B., Sihag, R.K., and Nixon, R.A. (1990). Dynamics of phosphorylation and assembly of the high molecular weight neurofilament protein subunit in NB2a/d1 neuroblastoma. J. Neurochem. 55:1784–1792.
Sobue, G., Hashizume, Y., Yasude, T., Mukai, E., Kumagai, T., Mitsuma, T., and Trojanowski, J.Q. (1990). Phosphorylated high molecular weight neurofilament protein in lower neurons in amyotrophic lateral sclerosis and other neurodegenerative diseases involving ventral horn cells. Acta Neuropathol. (Berl.) 79:402–408.
Smith, Q.R., Momma, S., Aoyagi, M., and Rapoport, S.I. (1987). Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 49:1651–1658.
Snyderman, S.E., Norton, P.M., and Roitman, B. (1964). Maple syrup urine disease, with particular reference to dietotherapy. Pediatrics 34:454–472.
Steele, R.D. (1986). Blood-brain barrier transport of the alpha-keto acid analogs of amino acids. Fed. Proc. 45:2060–2064.
Sternberger, L.A., and Sternberger, N.H. (1983). Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad. Sci. USA 80:6126–6130.
Strong, M.J., Strong, W.L., Jaffe, H., Traggert, B., Sopper, M.M., and Pant, H.C. (2001). Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J. Neurochem. 76:1315–1325.
Tetzlaff,W., Bisby, M.A., and Kreutzberg, G.W. (1988). Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J. Neurosci. 8:3181–3189.
Trojanowski, J.Q., Schmidt, M.L., Shin, R.-W., Bramblett, G.T., Rao, D., and Lee, V.M. (1993). Altered tau and neurofilament proteins in neurodegenerative diseases: Diagnostic implications for Alzheimer's disease and Lewy body dementias. Brain Pathol. 3:45–54.
Tsuda, M., Tashiro, T., and Komiya, Y. (2000). Selective solubilization of high-molecular-mass neurofilament subunit during nerve regeneration. J. Neurochem. 74:860–868.
Vaydia, V.A., Terwilliger, R.Z., and Duman, R.S. (2000). Alterations in heavy and light neurofilament proteins in hippocampus following chronic ECS administration. Synapse 35:137–143.
Wong, P.C., Marszalek, J., Crawford, T.O., Xu, Z., Hsieh, S.-T., Griffin, J.W., and Cleveland, D.W. (1995). Increasing neurofilament subunit NF-M expression reduces axonal NF-H, inhibits radial growth, and results in neurofilamentous accumulation in motor neurons. J. Cell Biol. 130:1413–1422.
Zhang, H., Sternberger, N.H., Rabinstein, L.J., Herman, M.H., Binder, L.I., and Sterberger, L.A. (1989). Abnormal processing of multiple proteins in Alzheimer disease. Proc. Natl. Acad. Sci. USA 86:8045–8049.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pessoa-Pureur, R., Funchal, C., de Lima Pelaez, P. et al. Effect of the Branched-Chain Alpha-Ketoacids Accumulating in Maple Syrup Urine Disease on the High Molecular Weight Neurofilament Subunit (NF-H) in Rat Cerebral Cortex. Metab Brain Dis 17, 65–75 (2002). https://doi.org/10.1023/A:1015459910869
Issue Date:
DOI: https://doi.org/10.1023/A:1015459910869