Abstract
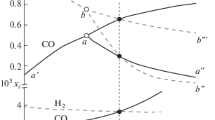
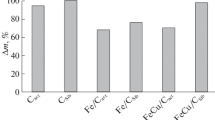
Computer simulations of equilibria in the Fe–C–NaCl–H2O–O2 system demonstrate that carbon has a significant influence on the composition of iron oxidation products. The effect of activated carbons with different physicochemical properties on the kinetics of heat generation during iron oxidation is studied. The process is shown to depend not only on the specific surface area and porosity of carbon but also on the stability of the complexes formed on the carbon surface via sorption of iron ions.
Similar content being viewed by others
REFERENCES
Yasuki, R. and Sahara, N., US Patent, 4649895, 1987.
Ishii, M., Kitasumi, K., and Sawara, N., Jpn. Patent 58-037075, 1981.
Usui, A., US Patent 5046479, 1991.
Usui, A., UK Patent Application 2297499A, 1996.
Usui, A., UK Patent Application 2303208A, 1997.
Sinyarev, G.B., Vatolin, N.A., Trusov, B.G., et al., Primenenie EVM dlya termodinamicheskikh raschetov metallurgicheskikh protsessov (Thermodynamic Computations in Metallurgy), Moscow: Nauka, 1982.
Vatolin, N.A., Moiseev, G.K., and Trusov, B.G., Termodinamicheskoe modelirovanie v vysokotemperaturnykh neorganicheskikh sistemakh (Thermodynamic Modeling in High-Temperature Inorganic Systems), Moscow: Metallurgiya, 1994.
Drobot, N.F., Pribylov, A.A., Ovchinnikova, N.A., et al., Effect of Physicochemical Characteristics of Activated Carbon on Water Sorption and Desorption, Zh. Fiz. Khim., 2001, vol. 75, no. 5, pp. 899-904.
Drobot, N.F., Gavrichev, K.S., Noskova, O.A., et al., Effect of Physicochemical Characteristics of Activated Carbon on Heat Generation during Oxidation of Fe-Con taining Mixtures, Zh. Fiz. Khim., 2002, vol. 76, no. 1, pp. 94-98.
Dubinin, M.M., Poristaya struktura i adsorbtsionnye svoistva aktivnykh uglei (Pore Structure and Adsorptive Properties of Activated Carbon), Moscow: VAKhZ, 1965.
Tarkovskaya, I.A., Okislennyi ugol' (Oxidized Carbon), Kiev: Naukova Dumka, 1981.
Dubinin, M.M., Nikolaev, K.M., Petukhova, G.A., et al., Effect of the Surface Chemistry of Activated Carbon on Water Vapor Adsorption, Izv. Akad. Nauk SSSR, Ser. Khim., 1991, no. 1, pp. 35-40.
Vartapetyan, R.Sh. and Voloshchuk, A.M., Mechanism of Water Adsorption by Carbon Adsorbents, Usp. Khim., 1995, vol. 64, no. 11, p. 1067.
Tarkovskaya, I.A., Stavitskaya, S.S., and Petrenko, T.P., Catalytic Activity of Different Oxidized Carbons in Redox Reactions, Adsorbts. Adsorbenty, 1979, no. 7, pp. 3–7.
Ripan, R. and Ceteanu, I., Chimia metalelor, Bucharest: Editura didactic si pedagogic, 1966. Translated under the title Neorganicheskaya khimiya, Moscow: Mir, 1972, vol. 2, p. 528.
Wesp, E.F. and Brode, W.R., The Ferric Chloride-Phenol Reaction, J. Am. Chem. Soc., 1934, vol. 56, p. 1037.
Hernandez-Apaolaza, L., Barak, P., and Lucena, J.J., Chromatographic Determination of Commercial Fe(III) Chelates of Ethylenediaminetetraacetic Acid, Ethylenediaminedi (?-hydroxyphenylacetic) Acid and Ethylenediaminedi (o-hydroxy-p-methylphenylacetic) Acid, J. Chromatogr., A., 1997, vol. 789, pp. 453-460.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drobot, N.F., Babievskaya, I.Z., Gavrichev, K.S. et al. Role of Activated Carbon in Chemical Interactions in the Fe–C–NaCl–H2O–O2 Heat-Generating System. Inorganic Materials 38, 501–506 (2002). https://doi.org/10.1023/A:1015431307781
Issue Date:
DOI: https://doi.org/10.1023/A:1015431307781