Abstract
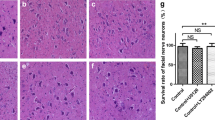
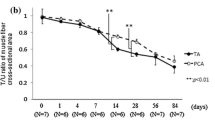
Testosterone propionate (TP) administration coincident with facial nerve injury accelerates the recovery rate from facial muscle paralysis in the hamster. One mechanism by which TP could augment peripheral nerve regeneration is through glial fibrillary acidic protein the facial motor nucleus. In a previous study, axotomy alone induces increases in GFAP mRNA, with TP significantly attenuating the axotomy-induced increases in GFAP mRNA. In the present study, immunoblotting techniques were used to extend our previous GFAP mRNA studies to the protein level. Castrated male hamsters were subjected to a right facial nerve transection, with half of the animals receiving subcutaneous implants of 100% crystalline TP. The left facial motor nucleus of each animal served as an internal control. Postoperative survival times include Days 4, 7, and 14. In non–TP-treated animals, facial nerve transections alone increased GFAP levels at all time points, relative to internal controls. As previously observed at the mRNA level, TP treatment attenuated but did not eliminate the axotomy-induced increase in GFAP levels at all time points tested. These results suggest that the regulatory actions of gonadal steroids on GFAP expression manifested in parallel at the mRNA/protein levels.
Similar content being viewed by others
REFERENCES
Aldskogius, H. (1982). Glial cell responses in the adult rabbit dorsal motor vagal nucleus during axon reaction. Neuropathol. Appl. Neurobiol. 8:341–349.
Blinzinger, K., and Kreutzberg, G. (1968). Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforsch. Mikrosk. Anat. 85:145–157.
Cammermeyer, J. (1955). Astroglial changes during retrograde atrophy of nucleus facialis in mice. J. Comp. Neurol. 102:133–150.
Day, J.R., Frank, A.T., O'Callaghan, J.P., Jones, B.C., and Anderson, J.E. (1998). The effect of age and testosterone on the expression of glial fibrillary acidic protein in the rat cerebellum. Exp. Neurol. 151:343–346.
Day, J.R., Laping, N.J., Lampert-Etchells, M., Brown, S.A., O'Callaghan, J.P</del>., McNeill, T.H., and Finch, C.E. (1993). Gonadal steroids regulate the expression of glial fibrillary acidic protein in the adult male rat hippocampus. Neuroscience 55:435–443.
Day, J.R., Laping, N.J., McNeill, T.H., Schreiber, S.S., Pasinetti, G., and Finch, C.E. (1990). Castration enhances expression of glial fibrillary acidic protein and sulfated glycoprotein-2 in the intact and lesion-altered hippocampus of the adult male rat. Mol. Endocrinol. 4:1995–2002.
Eng, L.F. (1988). Astrocytic response to injury. In (P. Reier, R. Bunge, and F. Seil, eds.), Current Issues in Neural Regeneration Research, Alan R Liss, New York.
Eng, L.F., Yu, A.C., and Lee, Y.L. (1992). Astrocytic response to injury. Prog. Brain Res. 94:353–365.
Ferreira, A., and Caceres, A. (1991). Estrogen-enhanced neurite growth: Evidence for a selective induction of Tau and stable microtubules. J. Neurosci. 11:392–400.
Garcia-Estrada, J., Del Rio, J.A., Luquin, S., Soriano, E., and Garcia-Segura, L.M. (1993). Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 628:271–278.
Goldstein, L.A., Kurz, E.M., and Sengelaub, D.R. (1990). Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J. Neurosci. 10:935–946.
Gould, E., Woolley, C.S., Frankfurt, M., and McEwen, B.S. (1990). Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 10:1286–1291.
Graeber, M.B., and Kreutzberg, G.W. (1986). Astrocytes increase in glial fibrillary acidic protein during retrograde changes of facial motoneurons. J. Neurocytol. 15:363–373.
Jones, K.J. (1993). Gonadal steroids and neuronal regeneration. A therapeutic role. Adv. Neurol. 59:227–240.
Jones, K.J., Durica, T.E., and Jacob, S.K. (1997a). Gonadal steroid preservation of central synaptic input to hamster facial motoneurons following peripheral axotomy. J. Neurocytol. 26:257–266.
Jones, K.J., Kinderman, N.B., and Oblinger, M.M. (1997b). Alterations in glial fibrillary acidic protein (GFAP) mRNA levels in the hamster facial motor nucleus: Effects of axotomy and testosterone. Neurochem. Res. 22:1359–1366.
Jones, K.J., and LaVelle, A. (1985). Changes in nuclear envelope invaginations in axotomized immature and mature hamster facial motoneurons. Brain Res. 353:241–249.
Jones, K.J., and Oblinger, M.M. (1994). Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J. Neurosci. 14:3620–3627.
Jones, K.J., Pfaff, D.W., and McEwen, B.S. (1985). Early estrogen-induced nuclear changes in rat hypothalamic ventromedial neurons: An ultrastructural and morphometric analysis. J. Comp. Neurol. 239:255–266.
Kinderman, N.B., and Jones, K.J. (1993). Testosterone enhancement of the nerve cell body response to injury: Evidence using in situ hybridization and ribosomal DNA probes. J. Neurosci. 13:1523–1532.
Krey, L.C., and McGinnis, M.Y. (1990). Time-courses of the appearance/disappearance of nuclear androgen C receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: Relationships with gonadotropin secretion. J. Steroid Biochem. 35:403–408.
Kujawa, K.A., Emeric, E., and Jones, K.J. (1991). Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J. Neurosci. 11:3898–3906.
Kujawa, K.A., and Jones, K.J. (1990). Testosterone-induced acceleration of recovery from facial paralysis in male hamsters: Temporal requirements of hormone exposure. Physiol. Behav. 48:765–768.
Kujawa, K.A., and Jones, K.J. (1995). Trophic actions of gonadal steroids on neuronal functioning normally and following injury. Adv. Neural. Sci. 2:131–152.
Kujawa, K.A., Kinderman, N.B., and Jones, K.J. (1989). Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp. Neurol. 105:80–85.
Laping, N.J., Nichols, N.R., Day, J.R., and Finch, C.E. (1991). Corticosterone differentially regulates the bilateral response of astrocyte mRNAs in the hippocampus to entorhinal cortex lesions in male rats. Brain Res. Mol. Brain Res. 10:291–297.
LaVelle, A., and LaVelle, F. (eds.) (1984). Neuronal reaction to injury during development. Early brain damage, neurobiology and behavior, Academic Press, New York.
Lieberman, A.R. (1971). The axon reaction: A review of the principal features of perikaryal responses to axon injury. Int. Rev. Neurobiol. 14:49–124.
Luine, V.N. (1985). Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp. Neurol. 89:484–490.
Matsumoto, A., Arnold, A.P., Zampighi, G.A., and Micevych, P.E. (1988a). Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J. Neurosci. 8:4177–4183.
Matsumoto, A., Micevych, P.E., and Arnold, A.P. (1988b). Androgen regulates synaptic input to motoneurons of the adult rat spinal cord. J. Neurosci. 8:4168–4176.
Nordeen, E.J., Nordeen, K.W., Sengelaub, D.R., and Arnold, A.P. (1985). Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science 229:671–673.
Norton, W.T., Aquino, D.A., Hozumi, I., Chiu, F.C., and Brosnan, C.F. (1992). Quantitative aspects of reactive gliosis: A review. Neurochem. Res. 17:877–885.
Oblinger, M.M., Kost, S.A., and Singh, L.D. (1993). Regulation of type III intermediate filament protein genes in astrocytes during development and after injury. New York.
Oblinger, M.M., and Singh, L.D. (1993). Reactive astrocytes in neonate brain upregulate intermediate filament gene expression in response to axonal injury. Int. J. Dev. Neurosci. 11:149–156.
O'Callaghan, J.P. (1991). Quantification of glial fibrillary acidic protein: Comparison of slot-immunobinding assays with a novel sandwich ELISA. Neurotoxicol. Teratol. 13:275–281.
Pfaff, D., and Keiner,M. (1973). Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol. 151:121–158.
Reier, P.J. (1986). Gliosis Following CNS injury: The anatomy of astrocytic scars and their influence on axonal elongation. In (S. Federoff and A. Vernadakis, eds.), Astrocytes: Cell Biology and Pathology of Astrocytes, Academic Press, Orlando, 3: pp. 263–324.
Reier, P.J., Eng, L.F., and Jakeman, L. (1989). Reactive astrocyte and axonal outgrowth in the injured CNS: Is gliosis really an impediment to regeneration. In (F.J. Siel, eds.), Neural Regeneration and Transplantation, Alan R. Liss, New York, 183–209.
Reier, P.J., Stensaas, L.J., and Guth, L. (1983). The astrocytic scar is an impediment to regeneration in the central nervous system. In (C.C. Kao, R.P. Bunge, and P.J. Reier, eds.), Spinal Cord Reconstruction, Raven Press, New York, 163–196.
Scheff, S.W., Morse, J.K., and DeKosky, S.T. (1988). Neurotrophic effects of steroids on lesion-induced growth in the hippocampus. I. The asteroidal condition. Brain Res. 457:246–250.
Simerly, R.B., Chang, C., Muramatsu, M., and Swanson, L.W. (1990). Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 294:76–95.
Tetzlaff, W., Bisby, M.A., and Kreutzberg, G.W. (1988). Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J. Neurosci. 8:3181–3189.
Yu, W.H., and McGinnis, M.Y. (1986). Androgen receptor levels in cranial nerve nuclei and tongue muscles in rats. J. Neurosci. 6:1302–1307.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coers, S., Tanzer, L. & Jones, K.J. Testosterone Treatment Attenuates the Effects of Facial Nerve Transection on Glial Fibrillary Acidic Protein (GFAP) Levels in the Hamster Facial Motor Nucleus. Metab Brain Dis 17, 55–63 (2002). https://doi.org/10.1023/A:1015415226799
Issue Date:
DOI: https://doi.org/10.1023/A:1015415226799