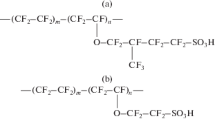Abstract
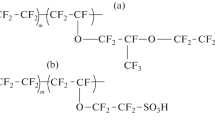
Transmembrane transport of ammonia and carbon dioxide through perfluorosulfonic membranes in ionic forms of transition metals was studied in a wide temperature interval. The different patterns of the temperature plots of the permeability coefficient of ammonia were found for different ionic forms of the membrane. An increase in the ammonia permeability with an increase in the moisture contents of the membrane also depends on its ionic form. The effects observed are explained by the different structures of water—ammonia complexes formed with metal ions. The mechanism of transmembrane transport of ammonia through perfluorosulfonic membranes in various ionic forms is discussed.
Similar content being viewed by others
References
A. M. Churakov, S. L. Ioffe, and V. A. Tartakovsky, Mendeleev Commun., 1991, 101.
A. M. Churakov, O. Yu. Smirnov, Yu. A. Strelenko, S. L. Ioffe, V. A. Tartakovsky, Yu. T. Struchkov, F. M. Dolgushin, and A. I. Yanovsky, Mendeleev Commun., 1994, 122.
A. M. Churakov, O. Yu. Smirnov, Yu. A. Strelenko, S. L. Ioffe, V. A. Tartakovsky, Yu. T. Struchkov, F. M. Dolgushin, and A. I. Yanovsky, Mendeleev Commun., 1996, 22.
A. E. Frumkin, A. M. Churakov, Yu. A. Strelenko, V. V. Kachala, and V. A. Tartakovsky, Org. Lett., 1999, 1, 721.
E. T. Apasov, A. M. Churakov, Yu. A. Strelenko, S. L. Ioffe, and V. A. Tartakovsky, Tetrahedron, 1995, 51, 6775.
A. M. Churakov, O. Yu. Smirnov, S. L. Ioffe, Yu. A. Strelenko, and V. A. Tartakovsky, Izv. Akad. Nauk, Ser. Khim., 1994, 1620 [Russ. Chem. Bull., 1994, 43, 1532 (Engl. Transl.)].
R. C. Zawalsky and P. Kovacic, J. Org. Chem., 1979, 44, 2130.
F. B. Mallory, K. E. Schueller, and C. S. Wood, J. Org. Chem., 1961, 26, 3312.
M. J. Mijs, S. E. Hoekstra, R. M. Ulmann, and E. Halinga, Rec. Trav. Chim. Pays-Bas, 1958, 77, 746.
R. R. Holmes and R. P. Bayer, J. Am. Chem. Soc., 1960, 82, 3454.
A. E. Frumkin, A. M. Churakov, Yu. A. Strelenko, and V. A. Tartakovsky, Izv. Akad. Nauk, Ser. Khim., 1999, 2126 [Russ. Chem. Bull., 1999, 48, 2103 (Engl. Transl.)].
S. G. Zlotin and O. A. Luk´yanov, Usp. Khim., 1993, 62, 157 [Russ. Chem. Rev., 1993, 62, 143 (Engl. Transl.)].
M. Hudlicky, Reduction in Organic Chemistry, Wiley, New York, 1984.
M. J. Kornet, W. Beaven, and T. Varia, J. Heterocycl. Chem., 1985, 22, 1089.
W. Gottardi, Monatsh. Chem., 1973, 104, 1681.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vorobiev, A.V., Beckman, I.N. Ammonia and carbon dioxide permeability through perfluorosulfonic membranes. Russian Chemical Bulletin 51, 275–281 (2002). https://doi.org/10.1023/A:1015403626398
Issue Date:
DOI: https://doi.org/10.1023/A:1015403626398