Abstract
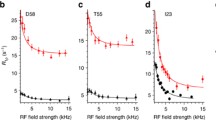
The impact of studying protein dynamics in supercooled water for identifying slow motional modes on the μs time scale is demonstrated. Backbone 15N spin relaxation parameters were measured at −13 °C for ubiquitin, which plays a central role for signaling proteolysis, cellular trafficking and kinase activation in eukaryotic organisms. A hitherto undetected motional mode involving Val 70 was found, which may well play an important role for ubiquitin recognition. The measurement of rotating frame 15N relaxation times as a function of the spin-lock field allowed determination of the correlation time of this motional mode, which would not have been feasible above 0 °C.
Similar content being viewed by others
References
Akke, M. and Palmer III, A.G. (1996) J. Am. Chem. Soc., 118, 911–912.
Akke, M., Brüschweiler, R. and Palmer III, A.G. (1993) J. Am. Chem. Soc., 115, 9832–9833.
Akke, M., Liu, J., Cavanagh, J., Erickson, H.P. and Palmer, A.G. (1998) Nat. Struct. Biol., 5, 55–59.
Blackledge, M.J., Brüschweiler, R., Griesinger, C., Schmidt, J.M., Xu, P. and Ernst, R.R. (1993) Biochemistry, 32, 10960–10974.
Carlomagno, T., Maurer, M., Hennig, M. and Griesinger, C. (2000) J. Am. Chem. Soc., 122, 5105–5113.
Cornilescu, G., Marquardt, J.L., Ottiger, M. and Bax, A. (1998) J. Am. Chem. Soc., 120, 6836–6837.
Cornilescu, G. and Bax, A. (2000) J. Am. Chem. Soc., 122, 10143–10154.
Davis, D.G., Perlman, M.E. and London, R.E. (1994) J. Magn. Reson., B104, 266–275.
de Alba, E., Baber, J.L. and Tjandra, N. (1999) J. Am. Chem. Soc., 121, 4282–4283.
Farrow, N.A., Muhandiram, R., Singer, A.U., Pascal, S.M., Kay, C.M., Gish, G., Shoelson, S.E., Pawson, T., Forman-Kay, J.D. and Kay, L.E. (1994) Biochemistry, 33, 5984–6003.
Feher, V.A and Cavanagh, J. (1999) Nature, 400, 289–293.
Fushman, D. and Cowburn, D. (1998) J. Am. Chem. Soc., 120, 7109–7110.
Haas, A.L. and Siepmann, T.J. (1997) FASEB J., 11, 1257–1268.
Ishima, R. and Torchia, D.A. (2000) Nat. Struc. Biol., 7, 740–743.
Kitahara, R., Yamada, H. and Akasaka, K. (2001) Biochemistry, 40, 13556–13563.
Meiler, J., Prompers, J.J., Peti, W., Griesinger, C. and Brüschweiler, R. (2001) J. Am. Chem. Soc., 123, 6098–6107.
Miura, T., Klaus, W., Gsell, B., Miyamoto, C. and Senn, H. (1999) J. Mol. Biol., 290, 213–228.
Orekhov, V.Y., Pervushin, K. and Arseniev, A.S. (1995) Eur. J. Biochem., 230, 887–896.
Ottiger, M. and Bax, A. (1998) J. Am. Chem. Soc., 120, 12334–12341.
Pickart, C. M. (2001) Mol. Cell, 8, 499–504.
Sakamoto, T., Tanaka, T., Ito, Y., Rajesh, S., Iwamoto-Sugai, M., Kodera, Y., Tsuchida, N., Shibata, T. and Kohno, T. (1999) Biochemistry, 38, 11634–11642.
Sandström, J. (1982) Dynamic NMR Spectroscopy. Academic Press, London.
Schneider, D.M., Dellwo, M.J. and Wand, A.J. (1992) Biochemistry, 31, 3645–3652.
Skalicky, J.J., Sukumaran, D.K., Mills, J.L. and Szyperski, T. (2000) J. Am. Chem. Soc., 122, 3230–3231.
Skalicky, J.J., Mills, J.L., Sharma, S. and Szyperski, T. (2001) J. Am. Chem. Soc., 123, 388–397.
Szyperski, T., Luginbühl, P., Otting, G., Güntert, P. and Wüthrich, K. (1993) J. Biomol. NMR, 3, 151–164.
Tjandra, N., Feller, S.E.; Pastor, R.W. and Bax, A. (1995) J. Am. Chem. Soc., 117, 12562–12566.
Tolman, J.R., Al-Hashimi, H.M., Kay, L.E. and Prestegard, J.H. (2001) J. Am. Chem. Soc., 123, 1416–1424.
Volkman, B.F., Lipson, D., Wemmer, D.E. and Kern, D. (2001) Science, 291, 2429–2433.
Wand, A.J. (2001) Nat. Struc. Biol., 8, 926–931. 67
Wilkinson, K.D., Laleli-Sahin, E., Urbauer, J., Larsen, C.N., Shih, G.H., Hass, A.L., Walsh, S.T. and Wand, A.J. (1999) J. Mol. Biol., 291, 1067–1077.
Yang D., Mok Y.K., Forman-Kay J.D., Farrow N.A. and Kay L.E. (1997) J. Mol. Biol., 272, 790–804.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mills, J.L., Szyperski, T. Protein dynamics in supercooled water: The search for slow motional modes. J Biomol NMR 23, 63–67 (2002). https://doi.org/10.1023/A:1015397305148
Issue Date:
DOI: https://doi.org/10.1023/A:1015397305148