Abstract
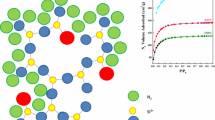
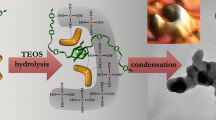
Four silica gels were prepared by hydrolysis of tetraethoxysilane (TEOS) in ethanol, using different catalysts: HCl, NaOH, NH3, and NBu4F. Nitrogen adsorption-desorption isotherms indicated that the HCl-catalyzed xerogel was purely microporous, whereas the other samples exhibited a very broad distribution of mesopores and a variable amount of micropores. 29Si MAS NMR spectroscopy of the wet gels (before drying) pointed to a low degree of condensation for the HCl-catalyzed gel, and to the presence of unhydrolyzed TEOS monomer in the NaOH-catalyzed gel. Comparison with the 29Si MAS NMR spectra of the xerogels indicated a significant increase of the degree of condensation during the drying procedure (3 hrs at 120°C under vacuum) for the HCl-catalyzed gel.
Similar content being viewed by others
References
C.J. Brinker and G.W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, 1990).
P. Judeinstein and C. Sanchez, J. Mater. Chem. 6, 511 (1996).
U. Schubert, J. Chem. Soc. Dalton Trans. 3343 (1996).
A. Vioux, Chemistry of Materials 9, 2292 (1997).
I.C. Tilgner, P. Fischer, F.M. Bohnen, H. Rehage, and W.F. Maier, Microporous Materials 5, 77 (1995).
R.L. Dumas, I. Tejedor-Tejedor, and M.A. Anderson, Journal of Porous Materials 5, 95 (1998).
L. Bourget, R.J.P. Corriu, D. Leclercq, P.H. Mutin, and A. Vioux, Journal of Non-Crystalline Solids 242, 81 (1998).
D.A. Loy and K.J. Shea, Chem. Rev. 95, 1431 (1995).
G. Cerveau, R.J.P. Corriu, C. Lepeytre, and P.H. Mutin, Journal of Materials Chemistry 8, 2707 (1998).
G. Cerveau, R.J.P. Corriu, and E. Framery, Polyhedron 19, 307 (2000).
T.J. Barton, L.M. Bull, W.G. Klemperer, D.A. Loy, B. McEnamey, M. Misono, P.A. Monson, G. Pez, G.W. Scherer, J.C. Vartuli, and O.M. Yaghi, Chem. Mater. 11, 2633 (1999).
C.J. Brinker, K.D. Keefer, D.W. Schaefer, R.A. Assink, B.D. Kay, and C.S. Ashley, J. Non-Cryst. Solids 63, 45 (1984).
T.W. Zerda, I. Artaki, and J. Jonas, J. Non-Cryst. Solids 81, 365 (1986).
W.G. Klemperer, V.V. Mainz, and D.M. Millar, Mater. Res. Soc. Symp. Proc. 73, 15 (1986).
J.C. Pouxviel, J.P. Boilot, J.C. Beloeil, and J.Y. Lallemand, J. Non-Cryst. Solids 89, 345 (1987).
W.G. Klemperer, V.V. Mainz, S.D. Ramamurthi, and F.S. Rosenberg, Mater. Res. Soc. Symp. Proc. 121, 15 (1988).
A.J. Vega and G.W. Scherer, J. Non-Cryst. Solids 111, 153 (1989).
F. Devreux, J.P. Boilot, F. Chaput, and A. Lecomte, Phys. Rev. A 41, 6901 (1990).
M. Mazur, V. Mlynarik, M. Valko, and P. Pelikan, Appl. Magn. Reson. 18, 187 (2000).
L. Malier, J.P. Boilot, F. Chaput, and F. Devreux, Phys. Rev. A 46, 959 (1992).
S. Brunauer, P.H. Emmett, and E.J. Teller, J. Am. Chem. Soc. 60, 309 (1938).
E.P. Barrett, L.S. Joyner, and P.P. Halenda, J. Am. Chem. Soc. 73, 373 (1951).
B.C. Lippens and J.H. deBoer, J. Catalysis 4, 319 (1965).
W.D. Harkins and G. Jura, J. Chem. Phys. 11, 431 (1943).
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquérol, and T. Siemienewska, Pure Appl. Chem. 57, 603 (1985).
A. Labrosse and A. Burneau, Journal of Non-Crystalline Solids 221, 107 (1997).
C. Dhalluin, C. Boutillon, A. Tartar, and G. Lippens, J. Am. Chem. Soc. 119, 10494 (1997).
W.G. Klemperer and S.D. Ramamurthi, Mater. Res. Soc. Symp. Proc. 121, 1 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Framery, E., Mutin, P. 29Si MAS-NMR Study of Silica Gels and Xerogels: Influence of the Catalyst. Journal of Sol-Gel Science and Technology 24, 191–195 (2002). https://doi.org/10.1023/A:1015391922572
Issue Date:
DOI: https://doi.org/10.1023/A:1015391922572