Abstract
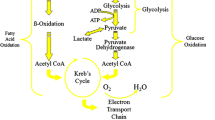
This review article discusses pharmacological approaches to optimizing myocardial metabolism during ischemia. Fatty acids are the main fuel for the healthy heart, with a lesser contribution coming from the oxidation of glucose and lactate. Myocardial ischaemia dramatically alters fuel metabolism, causing an accelerated rate of glucose conversion to lactate and a switch from lactate uptake by the heart to lactate production. This causes a dramatic disruption in cell homeostasis (e.g. lactate accumulation and a decrease in pH and ATP). Paradoxically, moderately ischemic tissue (∼50% of normal flow) continues to derive most of its energy (50–70%) from the oxidation of fatty acids despite a high rate of lactate production. This ischaemia-induced disruption in cardiac metabolism can be minimized by metabolic agents that reduce fatty acid oxidation and increase the combustion of glucose and lactate, resulting in clinical benefit to the ischemic patient. Agents that inhibit fatty acid beta-oxidation, such as ranolazine and trimetazidine, have proven to be effective in the treatment of stable angina. Treatment of acute myocardial infarction patients with an infusion of the glucose-insulin-potassium, which results in suppression of myocardial fatty acid oxidation and greater glucose combustion, has proven effective in reducing mortality. These metabolic therapies are free of direct hemodynamic or chronotropic effects, and thus are well positioned for use alongside traditional agents such as beta-adrenergic receptor antagonists or calcium channel antagonists.
Similar content being viewed by others
References
Vetter JR, Strange RC, Adams W, Oliver MF. Initial metabolic and hormonal response to acute myocardial infarction. Lancet 1974;1:284–289.
Liedtke AJ. Alterations in carbohydrate and lipid metabolism in the acutely ischaemic heart. Prog Cardiovasc Dis 1981;23:321–326.
Casademont J, Miro O. Electron transport chain defects in heart failure. Heart Failure Rev 2002; 7:131–139.
Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Failure Rev 2002;7:175–185.
Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Failure Rev 2002;7:115–130.
Stanley WC, Hoppel CL. Mitochondrial dysfunction in heart failure: potential for theraputic interventions? Cardiovasc Res 2000;45:805–806.
Werner JC, Whitman V, Vary TC, Fripp RR, Musselman J, Schuler HG. Fatty acid and glucose utilization in isolated, working newborn pig hearts. Am J Physiol 1983;244:E19–E23.
Kantor PF, Robertson MA, Coe JY, Lopaschuk GD. Volume overload hypertrophy of the newborn heart slows the maturation of enzymes involved in the regulation of fatty acid metabolism. J Am Coll Cardiol 1999;33:1724–1734.
Makinde AO, Gamble J, Lopaschuk GD. Upregulation of 5'-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res 1997;80:482–489.
Steinberg D, Khoo JC. Hormone-sensitive lipase of adipose tissue. Fed Proc 1977;36:1986–1990.
Lassers BW, Kaijser L, Carlson LA. Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3Hpalmitate. Eur J Clin Invest 1972;2:348–358.
Vyska K, Meyer W, Stremmel W, et al. Fatty acid uptake in normal human myocardium. Circ Res 1991;69:857–870.
Boden G, Chen X, Ruiz J, White JV, Rosetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 1994;93:2438–2446.
Lopschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 1993; 264:135–144.
Mjøs OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest 1971;50:1386–1389.
Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res 1996;79:940–948.
Liu B, el Alaoui-Talibi Z, Clnachan AS, Schulz R, Lopaschuk GD. Uncoupling of contractile function from mitochondrial TCA cycle activity and MVO2 during reperfusion of ischemic hearts. Am J Physiol 1996;270(1 Pt 2):H72–H80.
Liedtke AJ, Nellis S, Neely JR. Effects of excess free fatty acids on mechanical and metabolic function in normal and ischemic myocardium in swine. Circ Res 1978;43:652–661.
Lopaschuk GD, Spafford MA, Davies NJ, Wall SR. Glucose and palmitate oxidation in isolated working rat hearts reperfused after a period of transient global ischemia. Circ Res 1990;66:546–553.
Mjos OD, Kjekshus JK, Lekven J. Importance of free fatty acids as a determinant of myocardial oxygen consumption and myocardial ischemic injury during norepinephrine infusion in dogs. J Clin Invest 1974;53:1290–1299.
Spector AA. Lipids, hormones, and atherogenesis. The transport and utilization of free fatty acid. Ann NY Acad Sci 1968;149:768–783.
Oliver MF, Kurien VA, Greenwood TW. Relation between serum-free-fatty acids and arrhythmias and death after acute myocardial infarction. Lancet 1968;1:710–714.
Cowan RE, Thomson RP, Kaye JP, Hall RJ. Plasma bilirubin and serum free fatty acids after myocardial infarction. Postgrad Med J 1981;57:9–12.
Mueller HS, Ayres SM. Metabolic responses of the heart in acute myocardial infarction in man. Am J Cardiol 1978;42:363–371.
Prakash R, Parmley WW, Horvat M, Swan HJ. Serum cortisol, plasma free fatty acids, and urinary cathcholamines as indicators of complications in acute myocardial infarction. Circulation 1972; 45:736–745.
Takano S. Genetic studies on the arrhythmia in acute myocardial infarction with special reference to serum free fatty acid level. Jpn Circ J 1976;40: 287–297.
Tansey MJ, Opie LH. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet 1983;2:419–422.
Flink EB, Brick JE, Shane SR. Alterations of longchain free fatty acid and magnesium concentrations in acute myocardial infarction. Arch Iten Med 1981;141:441–443.
Kurien VA, Oliver MF. Serum-free-fatty-acids after acute myocardial infarction and cerebral vascular occlusion. Lancet 1966;2:122–127.
McDaniel HG, Papapietro SE, Rogers WJ, et al. Glucose-insulin-potassium induced alterations in individual plasma free fatty acids in patients with acute myocardial infarction. Am Heart J 1981;102: 10–15.
Gazes PC, Richardson JA, Woods EF. Plasma catechol amine concentrations in myocardial infarction and angina pectoris. Circulation 1959;19:657–661.
Porte D, Robertson RP. Control of insulin secretion by catecholamines, stress, and the sympathetic nervous system. Fed Proc 1973;32:1792–1796.
Svensson S, Svedjeholm R, Ekroth R, et al. Uptake of substrates and effects of insulin early after cardiac operations. J Thoracic Cardiovasc Surg 1990;99: 1063–1073.
Lopaschuk GD, Collins-Nakai R, Olley PM, et al. Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J 1994;128:61–67.
Gjesdal K. Platelet function and plasma free fatty acids during acute myocardial infarction and severe angina pectoris. Scand J Haemato 1976;17: 205–212.
Ceremuzynski L, Lada W, Matwiszyn B, Lawecki J. Patients with intractable angina: free thyroxine index, immunoreactive insulin and free fatty acids in blood, free adrenaline and noradrenaline in urine. Eur J Cardiol 1978;7:353–365.
Kleinfeld AM, Prothro D, Brown DL, Davis RC, Richieri GV, DeMaria A. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol 1996;78:1350–1354.
Kaijser L, Ericsson M, Walldius G. Myocardial turnover of plasma free fatty acids during angina pectoris by atrial pacing. Clin Physiol 1988;8:267–286.
Dagenais GR, Jalbert B. Effect of increased free fatty acids on myocardial oxygen extraction and angina threshold during atrial pacing. Circulation 1977; 56:315–319.
Dagenais GR, Gailis L, Julien P. Effects of pacinginduced ischemia on myocardial free fatty acid extractions. Clin Invest Med 1979;2:23–27.
Loogna E, Kaijser L, Carlson LA. Effect of plasma free fatty acid lowering on exercise tolerance and ST segment depression in patients with angina pectoris. Acta Med Scand 1979;206:371–374.
MacGowan GA, Casey M, Stirling R, Brett M, Kinsella A, Horgan JH. Exercise-related potassium and free fatty acid level changes in coronary artery Metabolic Treatment of Heart Disease 199 disease. Responses after moderate intensity training. Chest 1993;103:728–734.
Luxton MR, Miller NE, Oliver MF. Antilipolytic therapy in angina pectoris. Reduction of exerciseinduced ST segment depression. Br Heart J 1976; 38(11):1204–1208.
Kamihara S, Yokota M, Iwase M, et al. Early detection of myocardial ischemia by myocardial free fatty acid extraction in patients with exercise-induced angina pectoris. Am J Cardiol 1989;64:180–185.
Jenkins DJ, Welbon TA, Goff DV. Free fatty acids, beta-hydroxybutyrate, and ischaemic heart-disease. Lancet 1970;1:865–866.
Urbaszek W, Heiland G, Pankau H, Modersohn D. Influence of coronary-effective substances on the concentration of nonesterified fatty acids in the serum of patients with angina pectoris. Z Gesamte Inn Med 1975;30:15–18.
Laughton CW, Ruddle DL, Bedord CJ, Alderman EL. Sera containing elevated nonesterified fatty acids from patients with angiographically documented coronary atherosclerosis cause marked lipid accumulation in cultured human arterial smooth musclederived cells. Atherosclerosis 1988;70:233–246.
Sodi-Pollares D, Testelli MR, Fishleder BL, Bisteni A, Medrano GA, DeMicheli A. Effects of an intravenous infusion of a potassium-glucose-insulin solution on the electrocardiographic signs of myocardial infarction. Am J Cardiol 1962;9:166–181.
Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischemia and arrhythmias. Lancet 1994;343:155–158.
Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res 1991;68:466–481.
Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction. An overview of randomized placebocontrolled trials. Circulation 1997;96(4):1152–1156.
Diaz R, Paolasso EA, Piegas LS, et al. Metabolic modulation of acute myocardial infarction: the ECLA Glucose-Insulin-Potassium Pilot Trial. Circulation 1998;98:2227–2234.
Ceremuzynski L, Budaj A, Czepiel A, et al. Low-dose glucose-insulin-potassium is ineffective in acute myocardial infarction: results of a randomized multicenter pol-GIK trial. Cardiovas Drugs Ther 1999; 13:191–200.
Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation 1997;95:313–315.
Bersin RM, Stacpoole PW. Dichloroacetate as metabolic therapy for myocardial ischemia and failure. Am Heart J 1997;134:841–855.
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochem Biophys Acta 1994;1213:263–276.
Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischemic conditions. Potential for pharmacological interventions. Cardiovas Res 1997;33:243–257.
Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 1993;268: 25836–25845.
Stanley WC, Hernandez LA, Spires DA, Bringas J, Wallace S, McCormack JG. Pyruvate dehydrogenase activity and malonyl-CoA levels in normal and ischemia swine myocardium: effects of dichloroacetate. J Mol Cell Cardiol 1996;28:905–914.
Wargovich TJ, Macdonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiol 1988;61:65–70.
McVeigh JJ, Lopaschuk GD. Dichloroacetate stimulation of glucose oxidation improves recovery of ischemia rat hearts. Am J Physiol 1990;259:H1079–H1085.
Racey-Burns LA, Burns AH, Summer WR, Shepherd RE. The effect of dichloroacetate on the isolated no flow arrested rat heart. Life Sci 1989;44:2015–2023.
Wahr JA, Childs KF, Bolling SF. Dichloroacetate enhances myocardial functional and metabolic recovery following global ischemia. J Cardiothor Vascular Anesth 1994;8:192–197.
Stacpoole PW. The pharmacology of dichloroacetate. Metabolism 1989;38:112–114.
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1994;1213:263–276.
Lassers BW, Wahlqvist ML, Kaijser L, Carlson LA. Effect of nicotinic acid on myocardial metabolism in man at rest and during exercise. J Appl Physiol 1972;33:72–80.
Nuutila P, Knuuti MJ, Raitakari M, Ruotsalainen U, Teras M, Vioppio-Pulkki L, Haaparantaa M, Solin O, Wegelius U, Vki-Jarvinen H. Effect of antilipolysis on heart and skeletal muscle glucose uptake in overnight fasted humans. Am J Physiol 1994;267:E941–E946.
Stone CK, Holden J, Stanley WC, Perlman SB. Effect of substrate availability upon cardiac glucose uptake. J Nuclear Med 1995;36:996–1002.
Rowe MJ, Dolder MA, Kirby BJ, Oliver MF. Effect of a nicotinic-acid analogu on raised plasma-free-fattyacids after acute myocardial infarction. Lancet 1973;ii:814–818.
Rowe MJ, Neilson JMM, Oliver MF. Control of ventricular arrhythmias during myocardial infarction by antilipolytic treatment using a nicotinic-acid analogue. Lancet 1975;i:295–300.
Russell DC, Oliver MF. Effect of antilipolytic therapy on ST segment elevation during myocardial ischaemia in man. British Heart J 1978;40:117–123.
Higgins AJ, Morville M, Burges RA, Gardiner DG, Page MG, Blackburn KJ. Oxfenicine diverts rat muscle metabolism from fatty acid to carbohydrate oxidation and protects the ischaemic rat heart. Life Sci 1980;27:963–970.
Kennedy JA, Unger SA, Horowitz JD. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharmacol 1996;52:273–280.
Bunde CA, Grupp IA, Grupp G. Effects of the antianginal agent perhexilene maleate on exercise induced tachycardia in human volunteers. Fed Proc 1969;28:672.
Hirshleifer I. Perhexiline maleate in the treatment of angina pectoris. Curr Ther Res Clin Exp 1969;11:99–105.
Brown MJ, Horowitz JD, Mashford ML. A doubleblind trial of perhexiline maleate in the prophylaxis of angina pectoris. Med J Aust 1976;1:260–263.
Pilcher J. Comparative trial of perhexiline maleate and oxprenolol in patients with angina pectoris. Postgrad Med J 1978;54:663–667.
Horgan JH, O'Callaghan WG, Teo KK. Therapy of angina pectoris with low-dose perhexiline. J Cardiovasc Pharmacol 1981;3:566–572.
Cole PL, Beamer AD, McGowan N, et al. Efficacy and safety of perhexiline maleate in refractory angina. A double-blind placebo-controlled clinical trial of a novel antianginal agent. Circulation 1990;81:1260–1270.
Pepine CJ, Schang SJ, Bemiller CR. Effects of perhexiline on symptomatic and hemodynamic responses to exercise in patients with angina pectoris. Am J Cardiol 1974;33:306–312.
Pepine CJ, Schang SJ, Bemiller CR. Effects of perhexiline on coronary hemodynamic and myocardial metabolic responses to tachycardia. Circulation 1974;49:887–893.
Hudak WJ, Lewis RE, Kuhn WL. Cardiovascular pharmacology of perhexiline. J Pharmacol Exp Ther 1970;173:371–382.
Fleckenstein-Grun G, Fleckenstein A, Byon YK, Kem KW. Mechanism of action of Ca+ + antagonists in the treatment of coronary disease, with special reference to perhexiline maleate. In Perhexiline Maleate. Proceedings of a Symposium. Amsterdam; Excerpta Medica, 1978:1–22.
Vaughn-Williams EM. Previous evidence concerning the mode of action of perhexiline. Anti-arrhythmic action and the puzzle of perhexiline. London; Academic Press, 1980.
Jeffrey FMH, Alvarez L, Diczku V, Sherry AD, Malloy CR. Direct evidence that perhexiline modifies myocardial substrate utilization from fatty acids to lactate. J Cardiovasc Pharmacol 1995;25:469–472.
Barry WH, Horowitz JD, Smith TW. Comparison of negative inotropic potency, reversibility and effects on calcium influx of six calcium antagonists in cultured myocardial cells. Br J Pharmacol 1985; 85:51–59.
Shah RR, Oates NS, Idle JR, Smith RL, Lockhart JDF. Impaired oxidation of debrisoquine in patients with perhexiline neuropathy. BMJ 1982;284:295–299.
Morgan MY, Reshef R, Shah RR, Oates NS, Smith RL, Sherlock S. Impaired oxidation of debrisoquine in patients with perhexiline liver injury. Gut 1984;25:1057–1064.
Wright GJ, Leeson GA, Zeiger AV, Lang JW. The absorption, excretion and metabolism of perhexiline maleate by the human. Postgrad Med J 1973;49 (Suppl 3):8–15.
Horowitz JD, Sia STB, Macdonald PS, Goble AJ, Louis WJ. Perhexiline maleate treatment for severe angina pectoris-correlations with pharmacokinetics. Int J Cardiol 1986;13:219–229.
Sloan TP, Mahgoub A, Lancaster R, Smith RL. Polymorphism of carbon oxidation of drugs and clinical implications. BMJ 1978;2:655–657.
Lü llman H, Lü llman-Rauch R. Perhexiline induces generalized lipidosis in rats. Klin Wschr 1978; 56:309–310.
Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemia injury independent of changes in long chain acylcarnitine. Circ Res 1988;63:1036–1043.
Lopaschuk GD, Spafford M, Davies, NJ, Wall, SR. Glucose and palmitate oxidation in isolated working rat hearts reperfused following a period of transient global ischemia. Circ Res 1990;66:546–553.
Vetter R, Rupp H. CPT-I inhibition by etomoxir has a chamber-related action on cardiac sarcoplasmic reticulum and isomyosins. Am J Physiol 1994; 267:H2091–H2099.
Zarain-Herzberg A, Rupp H, Elimban V, Dhalla NS. Modification of sarcoplasmic reticulum gene expression in pressure overload cardiac hypertrophy by etomoxir. FASEB J 1996;10:1303–1309.
Rupp H, Elimban V, Dhalla NS. Modification of subcellular organelles in pressure-overloaded heart by etomoxir, a carnitine palmitoyltransferase I inhibitor. FASEB J 1992;6:2349–2353.
Turcani M, Rupp H. Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation 1997;96:3681–3686.
Turcani M, Rupp H. Modification of left ventricular hypertrophy by chronic etomoxir treatment. Br J Pharmacol 1999;126:501–507.
Bristow M. Etomoxir: a new approach to treatment of chronic heart failure. Lancet 2000;356:1621–1622.
Schmidt-Schweda S, Holubarsch. First clincial trial with etomoxir in patients with chronic congestive heart failure. Clin Sci 2000;99:27–35.
Barnish IT, Cross PE, Danilewicz JC, Dickinson RP, Stopher DA. Promotion of carbohydrate oxidation in the heart by some phenylglyoxylic acids. J Med Chem 1981;24:399–404.
Drake-Holland AJ, Passingham JE. The effect of Oxfenicine on cardiac carbohydrate metabolism in intact dogs. Basic Res Cardiol 1983;78:19–27.
Higgins AJ, Morville M, Burges RA, Gardiner DG, Page MG, Blackburn KJ. Oxfenicine diverts rat muscle metabolism from fatty acid to carbohydrate oxidation and protects the ischemia rat heart. Life Sci 1980;27:963–970.
Higgins AJ, Morville M, Burges RA, Blackburn KJ. Mechanism of action of oxfenicine on muscle metabolism. Biochem Biophys Res Comm 1981;100:291–296.
Burges RA, Gardiner DG, Higgins AJ. Protection of the ischaemic dog heart by oxfenicine. Life Sci 1981;29:1847–1853.
Stephens TW, Higgins AJ, Cook GA, Harris RA. Two mechanisms produce tissue-specific inhibition of fatty acid oxidation by oxfenicine. Biochem J 1985; 227:651–660.
Bielefeld DR, Vary TC, Neely JR. Inhibition of carnitine palmitoyl-CoA transferase activity and fatty acid oxidation by lactate and oxfenicine in cardiac muscle. J Mol Cell Cardiol 1985;17:619–625.
Vik-Mo H, Mjos OD, Neely JR, et al. Limitation of myocardial infarct size by metabolic interventions that reduce accumulation of fatty acid metabolites in ischemic myocardium. Am Heart J 1986; 111: 1048.
Bellemin-Baurreau J, Poizot A, Hicks PE, Armstrong JM. An in vitro method for the evaluation of antiarrhythmic and antischemic agents by using programmed electrical stimulation of rabbit heart. J Pharmacol Toxicol Methods 1994;31(1):31–40.
Yamada KA, McHowat J, Yan G, et al. Cellular uncoupling induced by accumulation of long-chain acetylcarnitine during ischemia. Circ Res 1994; 74:83–95.
Bergman G, Atkinson L, Metcalfe J, Jackson J, Jewitt DE. Beneficial effect of enhanced myocardial carbohydrate utilisation after oxfenicine (L-hydroxyphenylglycine) in angina pectoris. Eur Heart J 1980; 1:247–253.
Kerner J. Hoppel C. Genetic disorders of carnitine metabolism and their nutritional management. Annual Rev Nutrition. 1998;18:179–206.
Broderick TL, Quinney HA, Lopaschuk GD. Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart. J Biol Chem 1992;267:3758–3763.
Broderick TL, Quinney, HA, Barker CC, Lopaschuk GD. Beneficial effect of carnitine on mechanical recovery of rat hearts reperfused after a transient period of global ischemia is accompanied by a stimulation of glucose oxidation. Circ 1993;87:972–981.
Lopaschuk GD. Abnormal mechanical function in diabetes: relationship to altered myocardial carbohydrate/ lipid metabolism. Coron Art Dis 1996;7:116–123.
Schönekess BO, Allard MF, Lopaschuk GD. Propionyl L-carnitine improvement of hypertrophied heart function is accompanied by an increase in carbohydrate oxidation. Circ Res 1995;77:726–734.
Pierpont MM, Breningstall GN, Stanley CA, Singh A. Familial carnitine transporter defect: a treatable cause of cardiomyopathy in children. Am Heart J 2000;139:S96–S106.
Rizos I. Three-year survival of patients with heart failure caused by dilated cardiomyopathy and Lcarnitine administration. Am Heart J 2000;139: S120–S123.
Colonna P, Illiceto S. Myocardial infarction and left ventricular remodeling: results of the CEDIM trial. Am Heart J 2000; 139:S124–S130.
Di Lisa F, Menabó R, Siliprandi N. L-propionylcarnitine protection in ischemic rat heart. Mol Cell Biochem 1989;88:169–173.
Paulson DJ, Traxler J, Schimdt M, Noonan J, Shug AL. Protection of the ischaemic myocardium by Lpropionylcarnitine: effects on the recovery of cardiac output after ischaemia and reperfusion, carnitine transport, and fatty acid oxidation. Cardiovasc Res 1986;20:536–541.
Russell RR, Mommessin JI, Taegtmeyer H. Propionyl-L-carnitine-mediated improvement in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol 1995;268:H441–447.
Siliprandi N, Di Lisa F, Pivetta A, Miotto G, Siliprnadi D. Transport and function of L-camitine and Lpropionylcarnitine relevance to some cardiomyopathies and cardiac ischemia. Z Kardiol 1987;76(Suppl 5):34–40.
Sassen LMA, Bezstarosti K, Van der Giessen WJ, Lamers JMJ, Verdouw PD. L-propionylcarnitine increases post-ichemic blood flow but does not affect recovery of the energy charge. Am J Physiol 1991;261:H172–H180.
Brevetti G, Perna S, Sabbà C, Martone VD, Condorelli M. Propionyl-l-carnitine in intermittent claudication: double-blind, placebo-controlled, dose titration, multicenter study. J Am Coll Cardiol 1995;26:1411–1416.
Brevetti G, Perna S, Sabbà C, et al. Superiority of Lpropionylcarnitine vs L-carnitine in improving walking capacity in patients with peripheral vascular disease: an acute, intravenous, double-blind, crossover study. Eur Heart J 1992;13:251–255.
Bartels GL, Remme WJ, Pillay M, Schönfeld DHW, Kruijssen DACM. Effects of L-propionylcarnitine on ischemia-induced myocardial dysfunction in men with angina pectoris. Am J Cardiol 1994;74:125–130.
Stanley WC. Cardiac energetics during ischaemia and the rationale for metabolic interventions. Coronary Art Dis 2001;12(suppl 1):s3–s7.
Dalla-Volta S, Maraglino G, Della-Valentina P, Viena P, Desideri A. Comparison of trimetazidine with nifedipine in effort angina: a double-blind, crossover study. Cardiovasc Drugs Ther 1990;4:853–860.
Detry JM, Sellier P, Pennaforte S, Cokkinos D, Dargie H, Mathes P. Trimetazidine: a new concept in the treatment of angina. Comparison with propranolol in patients with stable angina. Br J Clin Pharmacol 1994;37:279–288.
Manchanda SC, Krischnaswami S. Combination treatemt with trimetazidine and diltiazem in stable angina pectoris. Heart 1997;78:353–357.
Sellier P. Chronic effects of trimetazidine on ergometric parameters in effort angina. Cariovasc Drugs Ther 1990;4:822–823.
Sellier P, Harpy C, Corona P, Audouin P, Ourbak P. Acute effects of trimetazidine on ergometric parameters in effort angina. Cardiovasc Drugs Ther 1990;4:820–821.
Szwed H, Hradec J, Preda I. Anti-ischemic efficacy and tolerability of trimetazidine administered to patients with angina pectoris: result of three studies. Coron Artery Dis 2001;12(Suppl 1):S25–S28.
McClella KJ, Plosker GL. Trimetazidine. A review of its use in stable angina pectoris and other coronary conditions. Drugs 1999;58:143–157.
Sellier P, Broustet JP. Efficacy at trough and safety of trimetazidine MR 35mg in patients with stable angina pectoris. Cardiovasc Drugs Therapy 2001; 15(Suppl 1):81.
El Banani H, Bernard M, Baetz D, Cabanes E, Coxxone P, Lucien A, Feuvray D. Changes in intra cellular sodium and pH during ischaemia-reperfusion are attenuated by trimetazidine. Comparison between low-and zero-flow ischaemia. Cardiovasc Res 2000;47:688–696.
Boucher FR, Hearse DJ, Opie LH. Effects of trimetazidine on ischemic contracture in isolated perfused rat hearts. J Cardiovasc Pharmacol 1994;24:45–49.
Fantini E, Demaison L, Sentex E, Grynberg A, Athias P. Some biochemical aspects of the protective effect of trimetazidine on rat cardiomyocytes during hypoxia and reoxygenation. J Mol Cell Cardiol 1994;26:949–958.
Lavanchy N, Martin J, Rossi A. Anti-ischemia effects of trimetazidine: 31P-NMR spectroscopy in the isolated rat heart. Arch Int Pharmacodyn 1987; 286:97–110.
Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 2000;86:580–588.
Lopaschuk GD. Optimizing cardiac energy metabolism: how can fatty acid and carbohydrate metabolism be manipulated? Coron Artery Dis 2001; 12(Suppl 1):s8-s11.
Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol 1996;28:341–350.
Clarke BMS, Patmore L, McCormack JG. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol 1993;109:748–750.
McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation 1996;93:135–145.
Allely MC, Brown CM, Kenny BA, Kilpatrick AT, Martin A, Spedding M. Modulation of alpha 1-adrenoceptors in rat left ventricle by ischaemia and acetyl carnitines: protection by ranolazine. J Cardiovasc Pharmacol 1993;21:869–873.
Gralinski MR, Black SC, Kilgore KS, Chou AY, McCormack JG, Lucchesi BR. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res 1994;28:1231–1237.
Schulz H. Personal Communication.
Cocco G, Rousseau MF, Bouvy T, et al. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J Cardiovasc Pharmacol 1992;20:131–138.
Pepine CJ, Wolff AA for the Ranolazine Study Group. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Am J Cardiol 1999;84:46–50.
Rousseau MF, Visser FG, Bax JJ, et al. Ranolazine: antianginal therapy with a novel mechanism: placebo controlled comparison versus atenolol. Eur Heart J 1994;15(Suppl):95.
Wolff AA for the MARISA Investigators. MARISA: Monotherapy assessment of ranolazine in stable angina. J Am Coll Cardiol 2000;35(Suppl A):408A.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wolff, A.A., Rotmensch, H.H., Stanley, W.C. et al. Metabolic Approaches to the Treatment of Ischemic Heart Disease: The Clinicians' Perspective. Heart Fail Rev 7, 187–203 (2002). https://doi.org/10.1023/A:1015384710373
Issue Date:
DOI: https://doi.org/10.1023/A:1015384710373