Abstract
Background. The subcutaneous (s.c.) administration of somatostatin analogs, such as octreotide acetate (SMS) and lanreotide, in patients with thyrotropin (TSH)-secreting pituitary adenomas (TSPA's)—thyrotropinomas with residual tumor after initial surgical therapy is effective in controlling hyperthyroidism, as well as curtailing tumor growth in the majority of patients. Long-acting preparations of the above agents, i.e. SMS-LAR and lanreotide-SR, have been synthesized and can be administered as depot injections intramuscularly (i.m.) at intervals of several weeks. Recent studies have reported on preliminary data regarding the use of such preparations in patients with TSPA's.
Materials and Methods: We present two cases of TSPA's with residual tumor following transsphenoidal adenomectomy. Neither of the two patients underwent external beam pituitary irradiation. The presence and extent of tumoral TSH hypersecretion was assessed by standard biochemical and dynamic endocrine testing, while tumor size was evaluated by conventional radiographic techniques.
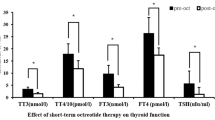
Results: In both patients, TSH secretion was effectively suppressed by SMS-LAR. Moreover, administration of this compound halted further tumor growth, as well as resulted in improved patient comfort, for 12 and 10 months respectively.
Conclusion: Our date corroborate earlier reports on the usefulness of SMS-LAR in the medical management of patients with TSPA's who have residual disease after initial pituitary surgery and/or irradiation.
Similar content being viewed by others
REFERENCES
McDermott MT, Ridgway EC. Central hyperthyroidism. Endocrinol Metab Clin North Am 1998;27:187–203.
McCutcheon IE, Weintraub BD, Oldfield EH. Surgical treatment of thyrotropin-secreting pituitary adenomas. J Neurosurg 1990;73:674–683.
Beck-Peccoz P, Mariotti S, Guillausseau PJ, et al. Treatment of hyperthyroidism due to inappropriate secretion of thyrotropin with the somatostatin analog SMS 201-995. J Clin Endocrinol Metab 1989;68:208–214.
Malarkey WB, Kovacs K, O'Dorisio TM. Response of a GH-and TSH-secreting pituitary adenoma to a somatostatin analogue (SMS 201-995): Evidence that GH and TSH coexist in the same cell and secretory granules. Neuroendocrinology 1989;49:267–274.
Shimatsu A, Murabe H, Kamoi K, et al. Treatment of thyrotropin-secreting pituitary adenomas with octreotide. Endocr J 1999;46:113–123.
Brucker-Davis F, Oldfield EH, Skarulis MC, et al. Thyrotropinsecreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab 1999;84:476–486.
Katznelson L, Oppenheim DS, Coughlin JF, et al. Chronic somatostatin analog administration in patients with alphasubunit-secreting pituitary tumors. J Clin Endocrinol Metab 1992;75:1318–1325.
Ridgway EC, Klibanski A, Martorana MA, et al. The effect of somatostatin on the release of thyrotropin and its subunits from bovine anterior pituitary cells in vitro. Endocrinology 1983;6:1937–1942.
Comi RJ, Gesundheit N, Murray L, et al. Response of thyrotropin-secreting pituitary adenomas to a longacting somatostatin analogue. N Engl J Med 1987;317:12–17.
Gorden P, Comi RJ, Maton PN, et al. NIH conference. Somatostatin and somatostatin analogue (SMS 201-995) in treatment of hormone-secreting tumors of the pituitary and gastrointestinal tract and non-neoplastic diseases of the gut. Ann Intern Med 1989;110:35–50.
Ilias I, Mastorakos G. Complete regression of a somatotropinsecreting adenoma with lanreotide monotherapy. Presse Med 2000;29:1818–1819 (in French).
Lamberts SW, Reubi J-C, Krenning EP. Somatostatin analogs in the treatment of acromegaly. Endocrinol Metab Clin North Am 1992;21:737–752.
Thapar K, Kovacs KT, Stefaneanu L, et al. Antiproliferative effect of the somatostatin analog octreotide on growth hormone-producing pituitary tumors: Results of a multicenter randomized trial. Mayo Clin Proc 1997;72:893–900.
Florio T, Thellung S, Arena S, et al. Somatostatin and its analog lanreotide inhibit the proliferation of dispersed human nonfunctioning pituitary adenoma cells in vitro. Eur J Endocrinol 1999;141:396–408.
Oppizzi G, Cozzi R, Dallabonzana D, et al. Scintigraphic imaging of pituitary adenomas: An in vivo evaluation of somatostatin receptors. J Endocrinol Invest 1998;21:512–519.
Colao A, Di Sarno A, Marzullo P, et al. New medical approaches in pituitary adenomas. Horm Res 2000;53(Suppl 3):76–87.
Broson-Chazot F, Houzard C, Ajzenberg C, et al. Somatostatin receptor imaging in somatotroph and non-functioning pituitary adenomas: Correlation with hormonal and visual responses to octreotide. Clin Endocrinol (Oxf) 1997;47:589–598.
Warnet A, Harris AG, Renard E, et al. A prospective multicenter trial of octreotide in 24 patients with visual defects caused by nonfunctioning and gonadotropin-secreting pituitary adenomas. French Multicenter Octreotide Study Group. Neurosurgery 1997;41:786–795.
Andersen M, Bjerre P, Schroder H, et al. In vivo secretory potential and the effect of combination therapy with octreotide and cabergoline in patients with clinically nonfunctioning pituitary tumors. Clin Endocrinol (Oxf) 2001;54:23–30.
Gillis JC, Noble S, Goa KL. Octreotide long-acting release (LAR). A review of its pharmacological properties and therapeutic use in the management of acromegaly. Drugs 1997;53:681–699.
Anthony LB. Long-acting formulations of somatostatin analogues. Ital J Gastroenterol Hepatol 1999;31(Suppl 2):S216–S218.
Lancranjan I, Bruns C, Grass P, et al. Sandostatin LAR: A promising therapeutic tool in the management of acromegalic patients. Metabolism 1996;45(Suppl 1):67–71.
Colao A, Ferone D, Marzullo P, et al. Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab 2001;86:2779–2786.
Flogstad AK, Halse J, Bakke S, et al. Sandostatin LAR in acromegalic patients: Long-term treatment. J Clin Endocrinol Metab 1997;82:23–28.
Taylor TJ, Donlon SS, Bale AE, et al. Treatment of a thyrotropinoma with octreotide-LAR in a patient with multiple endocrine neoplasia-1. Thyroid 2000;10:1001–1007.
Kuhn JM, Arlot S, Lefebvre H, et al. Evaluation of the treatment of thyrotropin-secreting pituitary adenomas with a slow release formulation of the somatostatin analog lanreotide. J Clin Endocrinol Metab 2000;85:1487–1491.
Caron P, Arlot S, Bauters C, et al. Efficacy of the longacting octreotide formulation (octreotide-LAR) in patients with thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab 2001;86:2849–2853.
Gancel A, Vuillermet P, Legrand A, et al. Effects of a slowrelease formulation of the new somatostatin analogue lanreotide in TSH-secreting pituitary adenomas. Clin Endocrinol (Oxf) 1994;40:421–428.
Saad B, Liu A, Brucker-Davis F, et al. Simplified screening test for resistance to thyroid hormone (RTH): The T3 challenge test (T3CT). In: Proc 77th Ann Mtg Endocr Soc 1995;#P1-396 (Abstract).
Beck-Peccoz P, Persani L, Mantovani S, et al. Thyrotropinsecreting pituitary adenomas. Metabolism 1996;45(Suppl 1):75–79.
Losa M, Magnani P, Mortini P, et al. Indium-111 pentetreotide single-photon emission tomography in patients with TSHsecreting pituitary adenomas: Correlation with the effect of a single administration of octreotide on serum TSH levels. Eur J Nucl Med 1997;24:728–731.
Shimon I, Yan X, Taylor JE, et al. Somatostatin receptor (SSTR) subtype-selective analogues differentially suppress in vitro growth hormone and prolactin in human pituitary adenomas. Novel potential therapy for functional pituitary tumors. J Clin Invest 1997;100:2386–2392.
Shimon I, Taylor JE, Dong JZ, et al. Somatostatin receptor subtype specificity in human fetal pituitary cultures. Differential role of SSTR2 and SSTR5 for growth hormone, thyroidstimulating hormone, and prolactin regulation. J Clin Invest 1997;99:789–798.
Chanson P, Boerlin V, Ajzenberg C, et al. Comparison of octreotide acetate LAR and lanreotide SR in patients with acromegaly. Clin Endocrinol (Oxf) 2000;53:577–586.
Kendall-Taylor P, Miller M, Gebbie J, et al. Long-acting octreotide LAR compared with lanreotide SR in the treatment of acromegaly. Pituitary 2000;3:61–65.
Turner HE, Vadivale A, Keenan J, et al. A comparison of lanreotide and octreotide LAR for treatment of acromegaly. Clin Endocrinol (Oxf) 1999;51:275–280.
Hunter SJ, Shaw JA, Lee Ko, et al. Comparison of monthly intramuscular injections of Sandostatin LAR with multiple subcutaneous injections of octreotide in the treatment of acromegaly; effects on growth hormone and other markers of growth SMS-LAR in Treatment of TSH-Secreting Tumors 143 hormone secretion. Clin Endocrinol (Oxf) 1999;51:245–251.
Iglesias P, Diez JJ. Long-term preoperative management of thyrotropin-secreting pituitary adenoma with octreotide. J Endocrinol Invest 1998;21:775–778.
Gesundheit N, Petrick PA, Nissim M, et al. Thyrotropinsecreting pituitary adenomas: Clinical and biochemical heterogeneity. Case reports and follow-up of nine patients. Ann Intern Med 1989;110:827–835.
Ando S, Sarlis NJ, Krishnan J, et al. Aberrant alternative splicing of thyroid hormone receptor in a TSH-secreting pituitary tumor is a mechanism for hormone resistance. Mol Endocrinol 2001;15:1529–1538.
Pernicone PJ, Scheithauer BW, Sebo TJ, et al. Pituitary carcinoma: A clinicopathologic study of 15 cases. Cancer 1997;79:804–812.
Kaltsas GA, Grossman AB. Malignant pituitary tumors. Pituitary 1998;1:69–81.
Mixson AJ, Friedman TC, Katz Da, et al. Thyrotropin-secreting pituitary carcinoma. J Clin Endocrinol Metab 1993;76:529–533.
Newman CB, Melmed S, George A, et al. Octreotide as primary therapy for acromegaly. J Clin Endocrinol Metab 1998;83:3034–3040.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gourgiotis, L., Skarulis, M.C., Brucker-Davis, F. et al. Effectiveness of Long-Acting Octreotide in Suppressing Hormonogenesis and Tumor Growth in Thyrotropin-Secreting Pituitary Adenomas: Report of Two Cases. Pituitary 4, 135–143 (2001). https://doi.org/10.1023/A:1015358721993
Issue Date:
DOI: https://doi.org/10.1023/A:1015358721993