Abstract
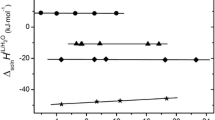
Real and chemical thermodynamic parameters of resolvation of the iodide ion in water–dimethyl sulfoxide mixtures at 298 K are determined by the method of Volta potential differences. The real parameters, as those of chloride and bromide ions earlier studied in similar solvents are positive, which is due to the restructuring of a surface layer at the solution/gas phase interface. An analysis of chemical energies of the iodide ion resolvation shows that anions that are capable of forming hydrogen bonds with proton-donor solvents are weakly solvated in aprotic solvents.
Similar content being viewed by others
REFERENCES
Krestov, G.A., Termodinamika ionnykh protsessov v rastvorakh (Thermodynamics of Ionic Processes in Solutions), Leningrad: Khimiya, 1984.
Mishchenko, K.P. and Poltoratskii, G.M., Termodinamika i stroenie vodnykh i nevodnykh rastvorov elektrolitov (Thermodynamics and Structure of Aqueous and Nonaqueous Electrolytes), Leningrad: Khimiya, 1976.
Conway, B.E., J. Solution Chem., 1978, vol. 7, p. 721.
Aleksandrov, V.V., Available from VINITI, 1974, Moscow, no. 225–74.
Rosseinsky, D.R., Chem. Rev., 1965, vol. 65, p. 467.
Izmailov, N.A., Elektrokhimiya rastvorov (The Electrochemistry of Solutions), Moscow: Khimiya, 1976.
Born, M., Z. Phys. Chem. (Leipzig), 1920, vol. 1, p. 45.
Atkinson, P.W. and McDermott, A.J., J. Chem. Educ., 1982, vol. 59, p. 359.
Gourary, B.S. and Adrian, F.J., Solid State Phys., 1960, vol. 10, p. 217.
Liszi, Y. and Ruff, J., The Chemical Physics of Solvation, Dogonadze, R.R. et al., Eds., Amsterdam: Elsevier, 1985, p. 119.
Krestov, G.A. and Abrosimov, V.K., Zh. Strukt. Khim., 1964, vol. 4, p. 510.
Noyes, R.M., J. Am. Chem. Soc., 1962, vol. 84, p. 513.
Volkov, A.G. and Kornyshev, A.A., Elektrokhimiya, 1985, vol. 21, p. 814.
Markin, V.S. and Volkov, A.G., Usp. Khim., 1987, vol. 56, p. 1353.
Popovich, O., Crit. Rev. Anal. Chem., 1970, vol. 1, p. 73.
Parker, A.J. and Alexander, R., J. Am. Chem. Soc., 1968, vol. 90, p. 3313.
Cox, B.G. and Parker, A.J., J. Am. Chem. Soc., 1973, vol. 95, p. 402.
Marcus, Y., Ion Solvation, New York: Wiley, 1985.
Grunvald, E., Baughman, G., and Kohnstam, G., J. Am. Chem. Soc., 1960, vol. 82, p. 5801.
Arnett, M. and McKelvey, O.R., J. Am. Chem. Soc., 1966, vol. 88, p. 2598.
Kim, Y.I., Bull. Soc. Chim. Belg., 1986, vol. 95, p. 435.
Stagert, Y. and Kamienska–Piotrowez, E., J. Chem. Soc., Faraday Trans. 1, 1997, vol. 93, p. 3463.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1990, vol. 26, p. 436.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1990, vol. 26, p. 429.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1995, vol. 31, p. 871.
Abrosimov, V.K., Korolev, V.V., Afanas'ev, V.N., et al., Eksperimental'nye metody khimii rastvorov: densimetriya, viskozimetriya, konduktometriya i drugie metody (Experimental Techniques in the Chemistry of Solutions: Densimetry, Viscosimetry, Conductimetry, and Other Methods), Moscow: Nauka, 1997, p. 186.
Parfenyuk, V.I., Paramonov, Yu.A., Chankina, T.I., and Krestov, G.A., Dokl. Akad. Nauk SSSR, 1988, vol. 302, p. 637.
Parfenyuk, V.I. and Paramonov, Yu.A., Elektrokhimiya, 1991, vol. 27, p. 781.
Parfenyuk, V.I. and Chankina, T.I., Zh. Fiz. Khim., 1998, vol. 72, p. 884.
Parfenyuk, V.I., Elektrokhimiya, 1999, vol. 35, p. 1469.
Parfenyuk, V.I. and Chankina, T.I., Elektrokhimiya, 1999, vol. 35, p. 1473.
Koltgoff, J., Pure Appl. Chem., 1971, vol. 25, p. 305.
Kenrick, F.B., Z. Phys. Chem. (Leipzig), 1896, vol. 19, p. 625.
Gordon, A.J. and Ford, R.A., The Chemist's Companion: A Handbook of Practical Data, Techniques and References, New York: Wiley, 1972.
Parfenyuk, V.I., Doctoral (Chem.) Dissertation, Ivanovo: Inst. Solution Chem., 2000.
Afanas'ev, V.N., Efremova, L.S., and Volkova, T.V., Fiziko–khimicheskie svoistva binarnykh rastvoritelei (Physicochemical Properties of Binary Solvents), Leningrad, 1988.
Parfenyuk, V.I. and Chankina, T.I., Zh. Fiz. Khim., 1996, vol. 70, p. 1330.
Parfenyuk, V.I. and Chankina, T.P., Zh. Fiz. Khim., 1997, vol. 71, p. 1707.
Abrosimov, V.K., in Sovremennye problemy khimii rastvorov (Current Problems in the Chemistry of Solvents), Berezin, B.D., Ed., Moscow: Nauka, 1986, p. 97.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parfenyuk, V.I., Chankina, T.I. Studying the Iodide Ion Solvation in Water–Dimethyl Sulfoxide Mixtures by the Method of Volta Potential Differences. Russian Journal of Electrochemistry 38, 431–434 (2002). https://doi.org/10.1023/A:1015300307626
Issue Date:
DOI: https://doi.org/10.1023/A:1015300307626