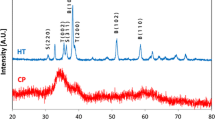
Abstract
The change in physicochemical properties of nanocrystalline powder of the composition ZrO2 ― 3 mole% Y2O3 in the presence of aluminum fluoride is studied. The starting powder is prepared by a complex method including elements of hydrothermal synthesis and sol-gel technology. It is established that these conditions expand the temperature limits for the existence of ZrO2 monoclinic solid solution. Transformation is connected with adsorption of fluorine at the ZrO2 surface, diffusion in the solid phase, and a reduction in anion vacancy concentration.
Similar content being viewed by others
References
Guo Gongyi, “Effect of preparation methods and condition of precursors on the phase composition of yttriastablized zirconia powders,” J. Amer. Ceram. Soc., 75,No. 5, 1294-1296 (1992).
G. Fisher, “Zirconia: ceramic engineering's toughness challenge,” Amer. Ceram. Soc. Bull., 65,No.10, 1355-1360 (1986).
F. M. Trubelja and V. S. Stubican, “Phase equilibria and ordering in the system zirconia -hafnia -yttria,” J. Amer. Ceram. Soc., 71,No.8, 662-666 (1988).
V. N. Strekalovskii, Yu. M. Polezhaev, and S. F. Pal'guev, Oxides and Impurity Disordering: Composition, Structure, Phase Transformations [in Russian], Nauka, Moscow (1987).
P. C. Rivas, J. A. Martiner, M. S. Caracoche, et al., “Evolution of the phase content of the zirconia powder prepared by sol-gel acid hydrolysis,” J. Amer. Ceram. Soc., 81,No.1, 200-204 (1998).
D. S. Rutman, Yu. S. Toropov, S. Yu. Pliner, et al., Highly Refractory Materials of Zirconium Dioxide [in Russian], Metallurgiya, Moscow (1985).
R. Goplan, C.-H. Chang, and V. S. Lin, “Thermal stability improvement on pore and phase structure of sol-gel derived zirconia,” J. Mat. Sci., 30, 3075-3081 (1995).
Feng-Chau_Wu and Shu-Chung Yu, “Effects of H2SO4 on the crystallization and phase transformation of zirconium powder in the precipitation processes,” J. Mat. Sci., 25,No.2A, 970-976 (1990).
C. Morterra, G. Cerreto, F. Pinna, and M. Signoretto, “Crystal phase spectral features and catalytic activity of sulfate-doped zirconia systems,” J. Catal., 157,No.1, 109-123 (1995).
D. M. Pasquevich, F. Lovey, and A. Caniero, “Structural and microstructural changes in zirconia in dilute chlorine atmosphere,” J. Amer. Ceram. Soc., 72,No.9, 1664-667 (1989).
T. Shigematsu, Y. Nakao, and Y. Nakanishi, “Effect of water on the tetragonal to monoclinic phase transformation of ZrO2 with Y2O3,” Sci. Sinter., 21,No.2, 73-79 (1989).
Yoshio Mursare and Etsuro Kato, “Role of water vapor in crystallite growth and tetragonal-monoclinic phase transformation of ZrO2,” J. Amer. Ceram. Soc., 66,No.3, 196-200 (1983).
A. K. Tjernlund, L. Hermansson, R. Carlsoson, and K. O. Axelsson, “Influence of combustion atmospheres on the phase transformation of zirconia,” J. Mat. Sci. Lett., 5,No.2, 129-131 (1986).
Y. Mashiro and M. Yoshimura, “Phase stability of zirconia,” Amer. Ceram. Soc. Bull., 67,No.12, 1950-1955 (1988).
M. Yoshimura, K. Kawabata, T. Noma, and S. Somiya, “Improvement of thermomechanical properties of YTZP by surface fluorination,” Proc. of the Annual Meeting of Ceramic Soc. of Japan, Vol.2, Nagoya (1987).
M. Yoshimura, Y. Okano, and S. Somiya, “Metastable tetragonal zirconia in the ZrO2 -YF3 -YO1.5 system,” Funtai Oyobi Fumatsu Yakin, 34,No.9, 441-444 (1987).
T. Mori, T. Kumaki, E. Kogura, et al., “Stability of zirconium dioxide of the tetragonal modification in molten metal fluorides,” Ege Kekaisi, 94,No.9, 961-969 (1986).
I. S. Kainarskii, E. V. Degtyarova, and I. G. Orlova, Corundum Refractories and Ceramics [in Russian], Metallurgiya, Moscow (1981).
E. V. Dudnik, A. V. Shevchenko, and A. K. Ruban, “Phase stability of materials based on ZrO2,” in: Contemporary Problems of Physical Materials Science, Part 1 [in Russian], Inst. Probl. Materials Sci., Ukrainian National Acad. Sci., Kiev (1999).
A. V. Shevchenko, A. K. Ruban, E. V. Dudnik, and V. A. Mel'nikova, “Hydrothermal synthesis of ultrafine zirconium dioxide powders,” Poroshk. Metall., Nos. 7-8, 74-80 (1997).
Jyung-Dong Lin and Jeng-Gong Duh, “Coprecipitation and hydrothermal synthesis of ultrafine 5.5 mole% CeO2 -2 mole% YO1.5 -ZrO2 powder,” J. Amer. Ceram. Soc., 80,No.1, 92-98 (1997).
R. Golan, C.-H. Chang, and Y. S. Lin, “Thermal stability improvement on pore and phase structure of sol-gel derived zirconia,” J. Mat. Sci., 30, 3075-3081 (1995).
F. F. Lange, “Transformation toughening. Part 1. Size effect associated with the thermodynamics of constrained transformations,” J. Mat. Sci., 17, 225-234 (1982).
R. C. Garvie and P. C. Nicholson, “Phase analysis in zirconia systems,” J. Amer. Ceram. Soc., 55,No.6, 303-305 (1972).
P. Duran, M. Villegas, J. F. Fernandez, et al., “Theoretically dense and nanostructural ceramics by pressureless sintering of nanosized Y-TZP powders,” Mat. Sci. Eng., A232, 168-176 (1997).
V. V. Skorokhod, Yu. M. Solonin, and I. V. Uvarova, Chemical, Diffusion, and Rheological Processes in Powder Material Technology [in Russian], Nauk. Dumka, Kiev (1990).
E. V. Dudnik, Z. A. Zaitseva, A. V. Shevchenko, and L. M. Lopato, “Sintering of ultrafine powders based on zirconium dioxide (Review),” Poroshk. Metall., Nos. 5-6, 43-52 (1995).
M. M. Godnev and L. L. Motov, Chemistry of Fluorine Compounds of Zirconium and Hafnium [in Russian], Nauka, Leningrad (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shevchenko, A.V., Dudnik, E.V., Ruban, A.K. et al. Change in the Physicochemical Properties of Nanocrystalline Powder Based on ZrO2 in the Presence of a Mineralizing Agent. Powder Metallurgy and Metal Ceramics 40, 544–551 (2001). https://doi.org/10.1023/A:1015244601207
Issue Date:
DOI: https://doi.org/10.1023/A:1015244601207